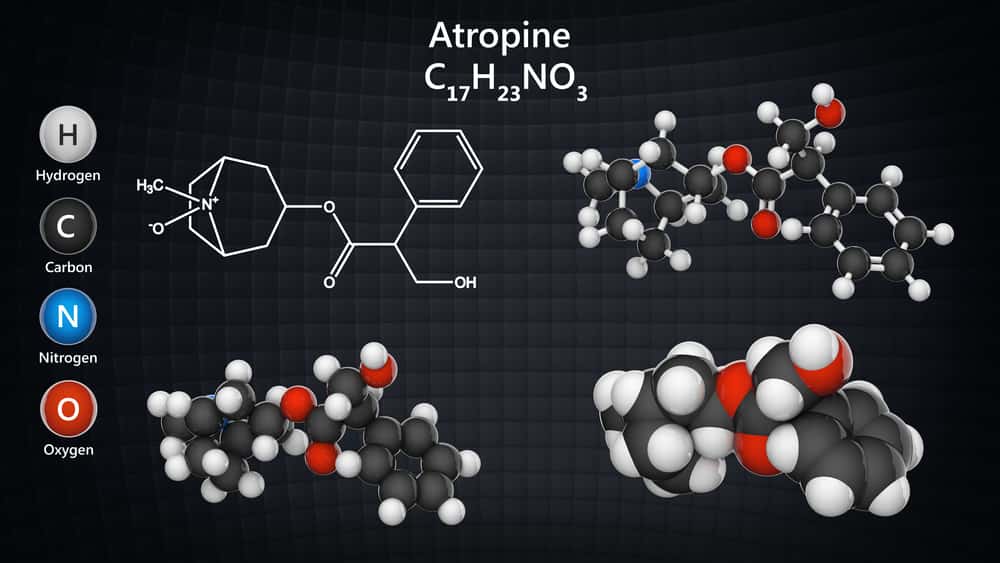
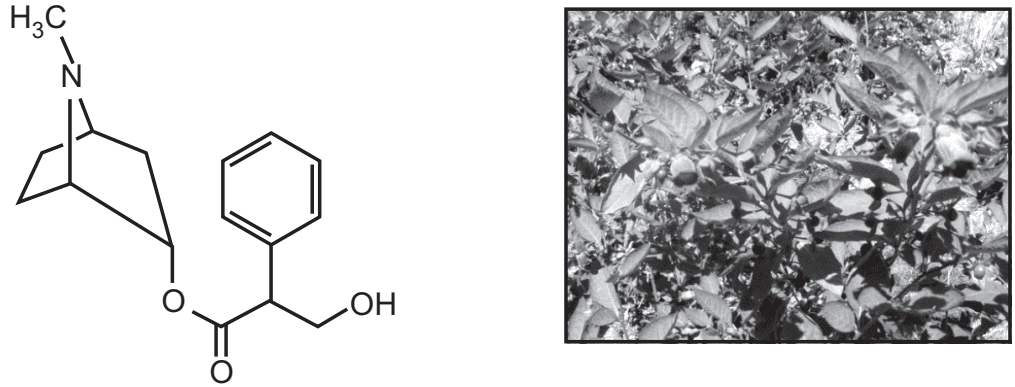
Atropine is an alkaloid isolated from the Hyoscyamus niger (leaf and flowering tops) or dried leaves of Hyoscyamus muticus of Atropa belladonna (aerial parts) or Atropa acuminata family Solanaceae. It is a poisonous product and its production in a synthetic way is costly compare to extraction from the natural way. It is a needle-like crystal, white colour or colourless, optically inactive usually present with laevorotary hyoscyamine. The melting point of atropine is 115-116°C.
Production
- The powdered drug is moistened with sodium carbonate aqueous solution and then it is extracted with benzene or ether. The residue is then extracted with acidified water. Remove the coloring matter by treating the aqueous extract with solvent ether.
- From this solution add sodium carbonate to precipitate the alkaloid. Wash the precipitate and dry it. Dissolve the dried precipitate in ether or acetone and dehydrate it using anhydrous sodium sulfate.
- Concentrate the filtrate and make it cool. After cooling the crystal of hyoscyamine and atropine are separated. Separate the crystal and dissolve in alcohol. To this solution add sodium hydroxide.
- Then allow standing this mixture. The crystal of hyoscyamine will be completely racemized in the atropine. For the purification of the crude atropine, dissolve it into acetone and then recrystallize it.
Estimation
Add alcohol in atropine sulfate and evaporate the alcohol. Dissolve the residue again in alcohol and add a calculated quantity of 0.1N HCl in this alcohol and titrate the excess of this acid with 0.1N NaOH using methyl red as an indicator. Each ml of 0.1 N HCl is equivalent to 0.3384 gm of atropine sulfate.
In another method atropine sulfate (around 0.1 gm) was weighed and dissolved in glacial acetic acid (50 ml). Titrate this solution with 0.1N perchloric acid and determine endpoint potentiometrically. Make the necessary correction by performing the blank determination.
Factor 1 ml of 0.1N HClO4 ≡ 0.0670 gm of atropine sulfate.
Utilization
Atropine sulfate acts as an anticholinergic drug. It is used in surgery as an anti-sialogogue to reduce nasal, salivary, bronchial, and pharyngeal secretion. It is dispensed through intramuscular injection before the anesthesia. It is used as an antidote for the poisoning of organophosphate insecticide and physostigmine. It also shows a mydriatic and antispasmodic property. In ophthalmology, it is also used to measure the refractive error.
Make sure you also check our other amazing Article on : Taxol