Biochemical Tests of Bacteria: Living microorganisms are differentiated based on various enzyme-catalysed metabolic reactions. The presence or absence of certain enzymes, intermediary metabolites, or end products often gives valuable information in identifying and classifying microorganisms.
(i) IMViC Test:
Table of Contents
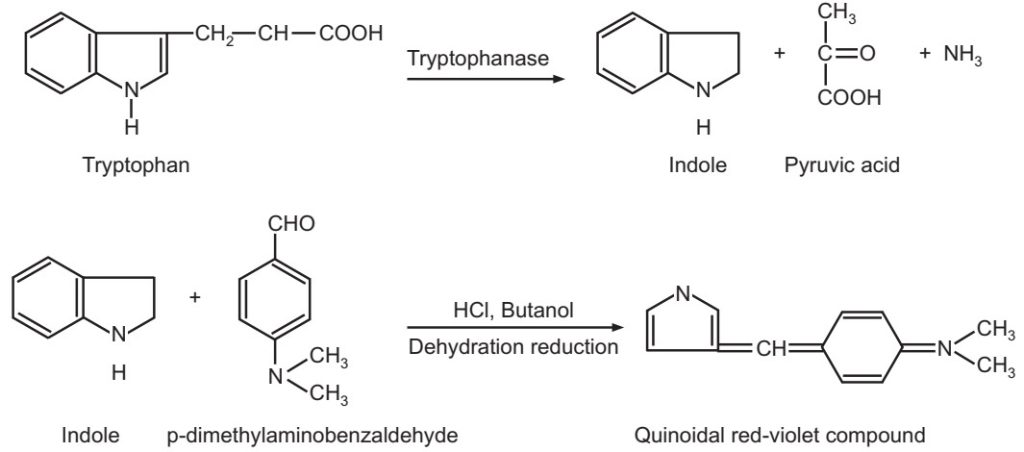
Indole production: Many bacterial species which possess the enzyme tryptophanase, degrade amino acid tryptophan to indole, pyruvic acid, and ammonia. Indole production is detected by inoculating the test microorganisms into peptone water and incubating it at 37 oC for 48-96 hours. Then add 0.5 ml of Kovac’s reagent and mix gently. The red colour in the alcohol layer indicates a positive reaction (production of indole).
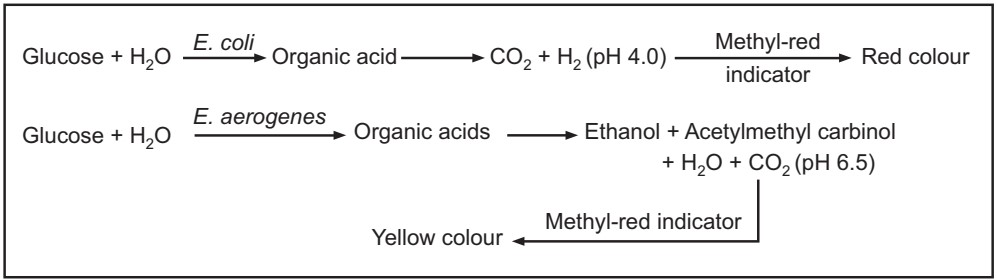
Methyl red (MR) test: This test is employed to detect the production of acid during the fermentation of glucose. By production of acid, the pH of the medium falls and it is maintained below 4.5. Inoculate the test microorganisms in glucose phosphate broth and incubate at 37 oC for 2 to 5 days. Then add five drops of 0.04% solution of methyl red, mix well and observe the result in the form of colour change. Red colour signifies a positive while yellow signifies a negative test.
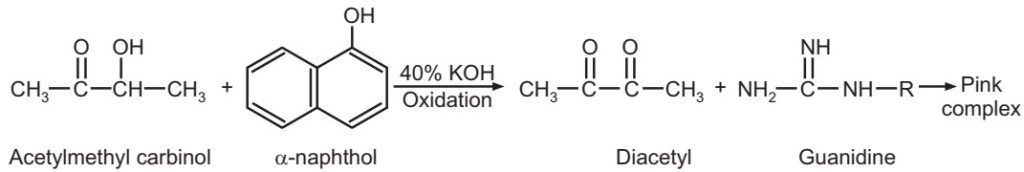
Voges – Proskauer (VP) test: Many bacteria ferment carbohydrates with the production of acetyl methyl carbinol (acetoin). In the presence of alkali and atmospheric oxygen, the small amount of acetyl methyl carbinol present in the medium is oxidised to diacetyl which reacts with the peptone of the broth to give a red colour.
Inoculate test microorganisms in glucose phosphate broth and incubate at 37 oC for 48 hours. Then add 1 ml potassium hydroxide and 3 ml of 5% solution of α- naphthol in absolute alcohol. A positive reaction is indicated by the development of pink colour in 2 to 3 minutes.
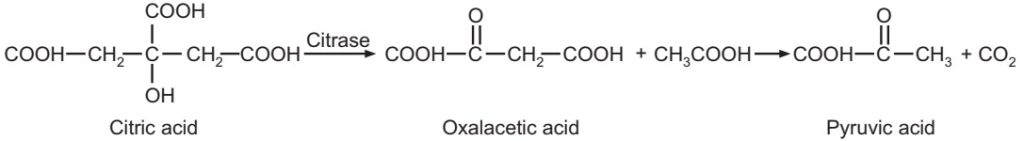
Citrate utilisation: This test is used to study the ability of microorganisms to utilise citrate as the sole source of carbon for growth. Koser’s citrate medium has citrate as the sole source of carbon. The ability of microorganisms to use citrate as the sole carbon source is indicated by the production of turbidity in the medium. Indole, MR, VP and citrate tests are done in routine for the classification of Gram-negative enteric bacteria. They are commonly referred to as IMViC tests.
(ii) Nitrate reduction test:
This test detects the production of the enzyme nitrate reductase which reduces nitrate to nitrite. Inoculate test microorganisms in a 5 ml medium containing potassium nitrate, peptone and distilled water. Incubate it at 37 oC for 96 hours. Then add 0.1 ml test reagent which consists of equal volumes of sulphanilic acid and α-naphthylamine in 5N acetic acid mixed just before use. A red colour developing within a few minutes indicates the presence of nitrate.
(iii) Hydrogen sulphide production:
Some microorganisms produce hydrogen sulphide from sulphur-containing amino acids. It may be detected by suspending strips of filter paper impregnated with lead acetate between the cotton plug and the tube. It has variable sensitivity. When cultured in media containing lead acetate or ferric ammonium citrate or ferrous acetate, they turn black or brown.
(iv) Potassium cyanide test:
This test is used to detect the ability of microorganisms to grow in the presence of potassium cyanide. Inoculate buffered peptone water medium containing 1 in 13,000 concentration of potassium cyanide with test microorganism. Incubate at 37 oC for 24-48 hours and detect the development of turbidity in the medium.
(v) Catalase production:
Add a loopful of 10% hydrogen peroxide on colonies of the test microorganisms on nutrient agar. Alternatively, pick up a few colonies of the test microorganisms with a platinum loop from the nutrient agar plate and dip it in a drop of 10% hydrogen peroxide on a clean glass slide. The production of gas bubbles from the culture indicates a positive reaction.
(vi) Urease test:
This test detects the ability of a microorganism to produce a urease enzyme. The test microorganism is inoculated on the entire slope of Christensen’s medium which contains urea and a phenol red indicator. It is incubated at 37 oC and examined after 4 hours and after overnight incubation. The development of the purple-pink colour indicates the production of urease. The latter in the presence of water converts urea into ammonia and carbon dioxide. Ammonia makes the medium alkaline and the phenol red indicator changes to purple-red.
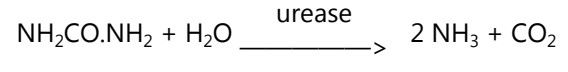
(vii) Oxidase test:
This test depends on the presence of oxidases (in bacteria) that catalyse the oxidation of reduced tetramethyl-p-phenylenediamine dihydrochloride (oxidase reagent) by molecular oxygen. Add a drop of freshly prepared 1% solution of oxidase reagent on a piece of filter paper. Then rub a few colonies of test microorganisms on it. If it is oxidase positive, it will produce a deep purple colour within 10 seconds. Alternatively, pour the oxidase reagent over the colonies of the test microorganism on the culture plate. The colonies of oxidase-positive microorganisms rapidly develop a deep purple colour.
(viii) Sugar fermentation:
The ability of microorganisms to ferment various sugars is tested by inoculation of the test microorganisms in different sugar media-containing indicators. Acid production is shown by a change in the colour of the medium to pink or red and the gas, if produced, gets collected in Durham’s tube.
(ix) Litmus milk reactions:
The major substrates capable of transformation are milk sugar lactose and the milk proteins casein, lactalbumin and lactoglobulin. Litmus milk forms an excellent differential medium in which microorganisms can metabolise milk substrates depending on their enzymatic complements such as lactose fermentation, gas production, litmus reduction, curd formation, proteolysis and alkaline reaction.
Make sure you also check our other amazing Article on : Principle and Procedure of Gram Staining