Chemical sterilization:
Table of Contents
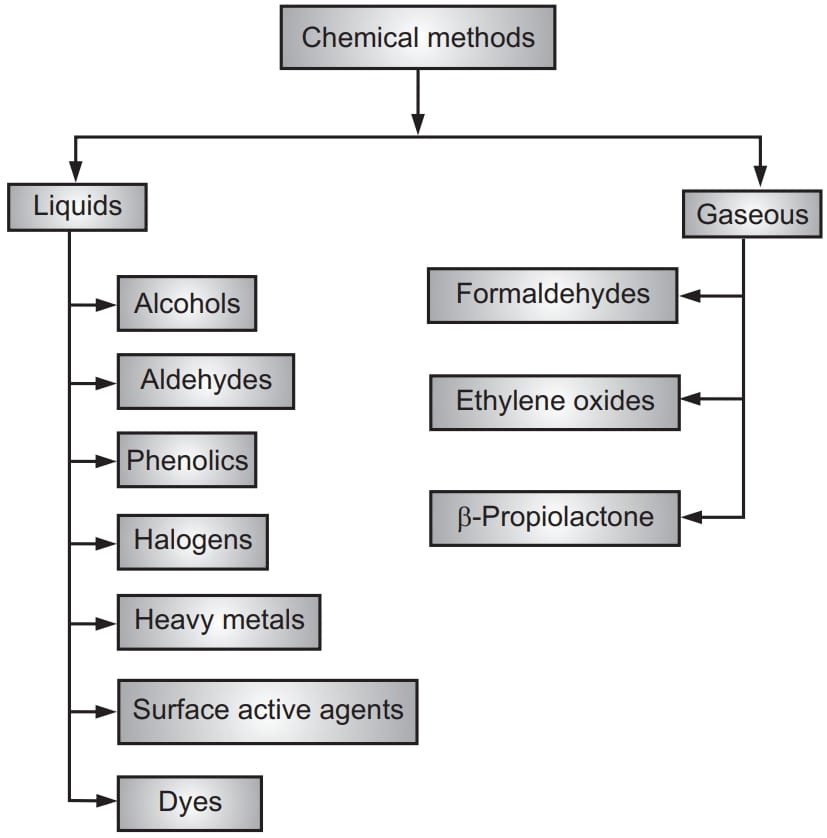
Disinfection is known as partial removal of pathogens whereas complete removal of microorganisms is called sterilization. Chemical sterilization is the process of elimination of all viable microorganisms and their spores. It is of two types viz. using liquids and gaseous compounds. Liquid sterilization involves submerging equipment in a chemical fluid for enough time to kill all viable microorganisms and their spores. Gas sterilization involves exposing equipment to chemical gases in an enclosed heated or pressurized chamber. Liquid sterilizing agents are impractical or ineffective for sterilizing items. Gaseous agents are more effective sterilants because they can permeate small openings and crevices easily. Gas chemicals also sterilize faster than liquids because they usually are combined with high heat. The gas residue is also easier to remove from sterilized articles but requires more expensive equipment.
Ideal Properties of Chemical Sterilization:
- A wide spectrum of activity.
- Active in presence of organic matter.
- Effective in acid as well as alkaline media.
- High penetration power.
- Stable.
- Speedy action.
- Compatible with other antiseptics and disinfectants.
- Safe and easy to use.
- Cheap and easily available.
- Not corrode metals.
Mode of Action of Chemical Disinfectants:
- It acts by protein coagulation.
- Disruption of cell membrane resulting in exposure, damage, or loss of the contents.
- Removal of free sulfhydryl group essential for the functioning of enzymes.
- Substrate competition: A compound resembling the essential substrate of the enzyme diverts the enzymes necessary for the metabolism of the cell and causes cell death.
Alcohol:
Mainly ethanol (80% v/v ethanol) or isopropyl alcohol (60-70% v/v) solutions are used to disinfect skin and decontaminate clean surfaces. Methanol is also used as a disinfectant but less bactericidal than ethanol and highly poisonous. Other alcohols such as propyl, butyl, amyl alcohols are more powerful germicidal than ethanol. Alcohols are effective against fungi, vegetative bacteria, mycobacterium species, and some lipid-containing viruses. They are more effective at concentration 70% in water. In tinctures, alcohols enhance the effectiveness of other antimicrobial chemicals.
Principle: It is based on denaturing coagulating proteins and dissolving membrane lipid of microorganisms.
Advantages:
- It is inexpensive and non-toxic.
- Widely available.
- Rapidly effective.
- Active against bacteria, viruses, mycobacterium.
Disadvantages:
- Not effective against bacterial spores.
- Not effective with organic materials.
Aldehydes:
They are low molecular weight compounds and act as antimicrobial. The most important two aldehydes are formaldehyde and glutaraldehyde. 2% solution of glutaraldehyde is known as Cidex which is used for bactericidal and viricidal in 10 minutes and sporicidal in 3-10 hours. Both these compounds are highly microbicidal and also kill spores. Formaldehyde is also a common aldehyde which is a gas in high concentration but at room temperature, it polymerizes and forms a solid substance. Formaldehyde solution as well as in gaseous form is used for sterilization and disinfection of enclosed areas respectively where vegetative cells are killed more quickly than spores. Formalin and paraformaldehyde are two important sources of formaldehyde when it is used for gaseous disinfection. Formalin is the aqueous solution of 40% formaldehyde. Formalin is extensively used for the preservation of specimens, inactive viruses, and bacteria in vaccines.
Principle:
Aldehyde combines with important proteins and nucleic acids of the bacterial cells. These interactions of an aldehyde with these cellular substances produce antimicrobial action. Formaldehyde inactivates microorganisms by alkylating the amino acids and sulfhydryl groups of proteins and ring nitrogen atoms of purine bases.
Advantage:
- They have good activity against spores, viruses, and fungi.
Disadvantages:
- They are toxic.
- They need a long exposure time for the action, a minimum of 3 hours.
- Freshness and pH are critical.
- They are irritant and carcinogenic.
- Glutaraldehyde is moderately active against TB.
Phenolics and Phenolic Agents:
Phenol or carbolic acid was first used by Lister as a disinfectant. Phenolics are the chemical derivatives of phenols. They are very effective disinfectants. Examples: cresols, bisphenols, etc. Cresols are derived from coal tar. A 5% aqueous solution of phenol rapidly kills the vegetative cells of microorganisms but spores are resistant. Phenols are used as a standard chemical to determine the antimicrobial activity of other similar chemical compounds by the Phenol coefficient method. This method is used for evaluating the effectiveness of disinfectants. Especially Staphylococcus aureus or Salmonella typhi are used for testing.
Hexylresorcinol is a phenolic derivative that is marketed in a solution of glycerin and water, acts as a strong surface tension reductant as well as high bactericidal activity. Pure crystalline phenol is colorless, its 5% aqueous solution is used as a disinfectant of sputum, urine, feces, etc. Quaternary ammonium compounds e.g. cetrimide are used in combination with other agents and acts as good detergent properties. Some other examples of phenolic compounds are amyl phenol, benzyl-4-chlorophenol, etc.
Principle:
They are having various modes of action. They act by destroying plasma membranes and denature proteins. They also affect plasma membrane, inactivate enzymes, and denature proteins. Some phenolics are mild enough for use as antiseptics.
Advantages:
- They are stable, persist for long times after application, and remain active in the presence of organic compounds.
- They are active against a wide range of organisms.
- They have good antimicrobial activity and are rapid bactericidal.
- They are more active in acid pH.
- They remain active on surfaces long after application.
Disadvantages:
- They are having caustic effects on the skin.
- They are having systemic toxicity.
- They are not effective against spores.
- They are not effective at low temperatures.
- They are incompatible with non-ionic and cation surfactants.
- They have a disagreeable odor
Halogens:
Halogens, mainly, iodine and chlorine are used as antimicrobial agents. Iodine is used as an antiseptic against all microbes, fungi, and viruses. It inhibits protein synthesis and oxidizes the sulphydryl group of amino acids. There are various preparations of iodine such as 2% iodine plus 2% sodium iodide diluted in alcohol, 7% iodine plus 5% potassium iodide in 83% alcohol, 5% iodine plus 10% potassium iodide in aqueous solution. Iodine is also used in the form of iodophors. Iodophors are mixtures of iodine with surface-active agents, used for the germicidal activity. Chlorine is used as a disinfectant. Hypochlorous acid (HOCl) is an aqueous solution of chlorine that is used as a disinfectant. Some other compounds like calcium hypochlorite [Ca(OCl)2], sodium hypochlorite (NaOCl), etc. are the preparation of chlorine. Chloramines are also chlorinated compound (chlorine and ammonia) which acts as antiseptics.
Principle:
Iodine is an oxidizing agent. These agents can irreversibly oxidize and inactivate essential metabolic compounds like proteins with sulphhydryl groups. It also has halogenations action of tyrosine unit of enzymes and other cellular proteins require tyrosine for activity.
Chlorinated compounds show antimicrobial activity due to the formation of hypochlorous acid in water. This hypochlorous acid is further decomposed into nascent oxygen which shows a strong oxidation reaction.
Advantages of Iodine compounds:
- Iodine compounds are effective against gram-positive bacteria.
- They produce residual activity.
- They retain microbial action in the presence of organic debris.
- They are available in solutions, sprays, and in-gel preparations.
Disadvantages of iodine compounds:
- Iodine compounds when used alone are major irritants.
- They are weak against mycobacteria, fungi, viruses.
- They are absorbed into the skin and can be toxic.
- They can cause 1st and 2nd degree burns.
Advantages of Chlorine Compounds:
- They are cheap and readily available.
- Chlorine is highly soluble in water.
- They leave a residue in the solution.
- They are toxic to most microorganisms.
- They remove iron and manganese and ammonia nitrogen during oxidation.
- They destroy the taste and other odor compounds.
Disadvantages of Chlorine Compounds:
- Chlorine is a poisonous and toxic gas.
- They are corrosive, require special non-metal conduits.
- They require careful handling, operation, and storage.
- The vapors of chlorine are irritant.
- They are strong oxidizing agents, react with most elements and compounds.
Heavy Metals:
Most heavy metals have a detrimental effect on microorganisms. Compounds such as mercury, silver, copper are more effective against microorganisms. Mercury compounds such as mercuric chloride, mercurous chloride, mercuric oxide are in dilution form act as bactericidal. They are highly toxic to animals but used in ointments as antiseptics. Some of the organic compounds of mercury are Mercurochrome, metaphor, mercerizing are used as antiseptics. They are fewer irritants and less toxic.
Silver compounds such as silver nitrate, silver lactate, silver picrate act as bacteriostatic as well as bactericidal when these metals are in contact with proteins of microorganisms. Silver nitrate is mainly used as antiseptics and other compounds are act as germicidal.
A copper compound such as copper sulfate is more effective against algae and molds than bacteria. 2 ppm in water prevents algal growth. It is used in the form of a Bordeaux mixture as a fungicide. Zinc salt controls infection caused by anaerobic bacteria.
Principle:
Heavy metals and their compounds have antimicrobial activity due to the combination with cellular proteins and inactivating their function. Example: mercuric chloride inhibits the action of the enzyme by acting on the sulfhydryl group of enzymes and form inactive enzymes. A high concentration of heavy metal salt coagulates cytoplasmic proteins of microorganisms that cause the death of the cells.
Advantages:
- They are powerful biocides.
- They form a complex with proteins of microorganisms and converts inactive form of microorganisms.
Disadvantage:
- They are highly toxic to the animals.
Surface Active Agents:
Soaps and detergents are mainly used as a surfactant. It depends on the alkali content in the soap how they show their action. They act as germicidal for pneumococci, streptococci, gonococci etc. Detergents are ionized in water and are made up of fats. They quickly dissolve in cold and hard water. Quaternary ammonium compounds are important cationic detergents that act as germicidal as well as bactericidal, mainly against Gram-positive bacteria. They also show fungicidal activity and destroy the pathogenic protozoa. Benzalkonium chloride is used as a disinfectant. Diaparene chloride acts as a bacteriostatic against Brevibacterium ammoniagenes.
Principle:
They denature proteins, interfere with glycolysis, and damage the membrane of the microorganisms. They also damage the cell cytoplasmic membrane and alter the cell structure.
Advantages:
- They are effective against vegetative bacteria.
- They are widely available.
- They are less expensive.
- They are non-irritant.
Disadvantages:
- They are ineffective against spores.
- They are not effective against non-enveloped viruses.
- They may become contaminated.
- They are easily inactivated by the presence of anionic detergents, soaps, and hard water.
Dyes:
There are two types of dyes exhibiting antimicrobial activity viz. triphenylmethane and acridine. Triphenylmethane dyes such as brilliant green, crystal violet, malachite green inhibit gram-positive bacteria and fungi. Malachite green is used to inhibit Staphylococcus aureus.
Acridine dyes are the compounds that are derived from acridine such as acriflavine, tryptoflavine, proflavine, etc. They inhibit bacteria especially Staphylococci and gonococci. Gonococci especially are inhibited by tryptoflavine. Acriflavine, proflavine are used to inhibit the growth of Gram-negative bacteria.
Principle: They have their inhibitory effect by interfering with cellular oxidation processes.
Advantages:
- They are bacteriostatic in high dilution.
- They are more active against Gram-positive bacteria.
Disadvantages:
- They are less active against Gram-negative bacteria.
- They have low bactericidal activity.
Gaseous Agents For Sterilization
Some important medical devices are required for sterilization but not possible through heat sterilization due to damage to the materials. In such cases, gaseous sterilization is required. Like Plastic syringes, blood transfusion apparatus, plastic pipettes, Petri dishes, etc. The main gaseous agents are used for sterilization are ethylene oxide, formaldehyde, beta-propiolactone, etc. They act on the principle of denaturing proteins and DNA by cross-linking functional groups. But this method has some disadvantages.
Disadvantages:
- Gas can be hazardous to people.
- They are often highly explosive.
- Some time gases are extremely poisonous.
- They are potentially carcinogenic.
Factors affecting gaseous sterilization:
- The efficiency of the sterilization method is influenced by the concentration of ethylene oxide.
- The humidity of the sterilizing atmosphere.
- The temperature of sterilization.
- Time of exposure.
- Physical nature and permeability of the load.
- Atmospheric preconditioning of the load before sterilization.
Formaldehydes:
It is also a group of alkylating agents. It inactivates microorganisms by alkylating the amino acid and sulfhydryl groups of proteins and ring nitrogen atoms of purine bases. It is a gaseous disinfectant and biocide. It is a strong, broad-spectrum disinfectant and biocide that can kill bacteria, viruses, fungi, and endospores. It is very irritating to live tissues and also carcinogenic, therefore it is not used as an antiseptic.
Ethylene Oxides:
It is a type of alkylating agent that is used for gaseous sterilization. It is highly penetrating and can sterilize items within plastic bags such as catheters, disposable items in laboratories and clinical settings.
- It is gaseous above 10.8°C.
- Ethylene oxide has high antimicrobial activity, it kills even endospores.
- It is used for the sterilization of heat-sensitive materials such as spices, oils, plastics, etc.
- Ethylene oxide is used in formulation with CO2 as Freon (CClFe).
β-Propiolactone:
It binds to DNA, thereby inactivating it. It is a clear liquid with a strong odor and can kill endospores. It has been used in either liquid form or as a vapor for the sterilization of medical instruments and tissue grafts, and it is a common component of vaccines, used to maintain their sterility. It is also used for the sterilization of nutrient broth, as well as blood plasma, milk, and water.
- It is gas above 15.5°C.
- Penetration power of β-propiolactone is less than ethylene oxide but it is more active in killing microorganisms.
- Due to its carcinogenic effects, it is not commonly used.
Applications of Gaseous Sterilization:
- This method is used for sterilizing thermolabile substances like hormones, proteins, various heat-sensitive drugs, etc.
Make sure you also check our other amazing Article on : Physical Methods of Sterilization