Chemical Synthesis and Reactions of Pyrrole: Pyrrole was first isolated from coal tar in 1834. It is an aromatic heterocycle having a weak aniline-like odor. It is a colorless volatile liquid which like aniline darken by autoxidation. It has a boiling point of 129 to 131°C.
Pyrrole has three pairs of delocalized π electrons. Two of the pairs are shown as the bonds and the third pair is shown as a lone pair of nonbonding electrons on the nitrogen atom.
Chemical Synthesis of Pyrrole
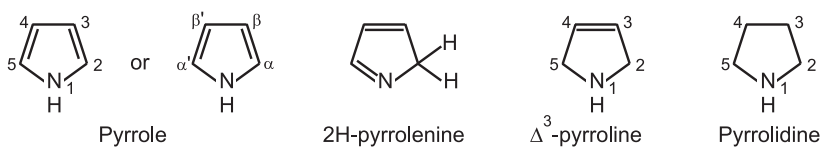
(1) Pyrrole is prepared industrially from furan by passing it over ammonia and steam and heated at 400°C in the presence of solid acid catalysts like SiO2 and Al2O3.
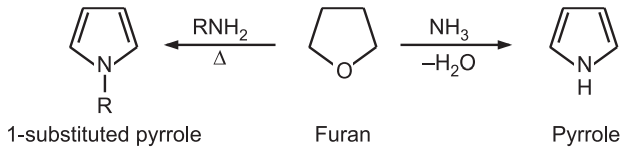
(2) Hantzsch Pyrrole synthesis: When α-halo ketone or aldehyde is reacted with a β-ketoester or β-chloromethane and a base like ammonia/primary amine, it gives pyrrole. The base functions both as a reactant and as a catalyst.
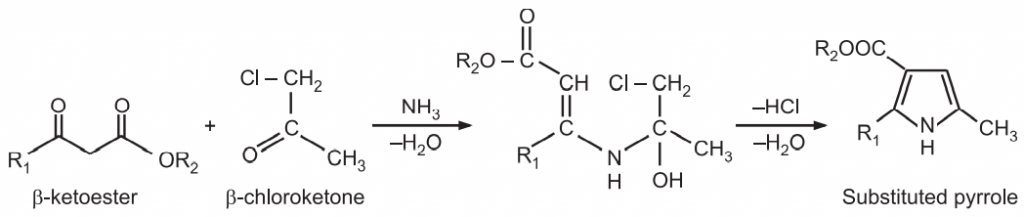
(3) Knorr Pyrrole Synthesis: In this widely used method, α – amino ketone is condensed with another dicarbonyl compound containing an electron-withdrawing group α to a carbonyl group (i.e., activated methylene group) in the presence of acetic acid.
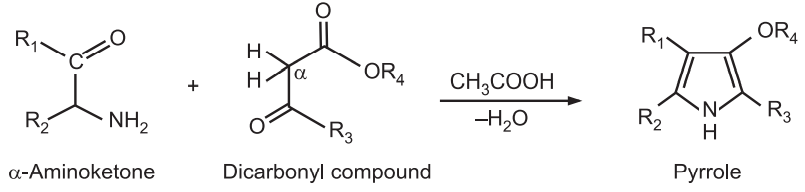
(4) Paal-Knorr Pyrrole Synthesis: It is a condensation reaction between 1,4-dicarbonyl compound with ammonia or a primary amine to form a substituted pyrrole. Pyrrole itself is formed from succinaldehyde and ammonia.
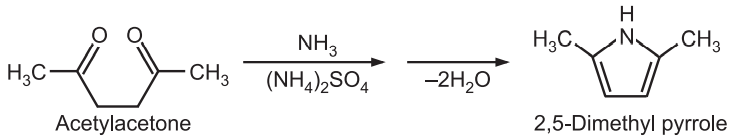
(5) Barton-Zard Synthesis: Pyrrole is obtained when an isocyanoacetate reacts with a nitroalkene in a 1, 4 – addition, followed by cyclization and elimination of nitro group.
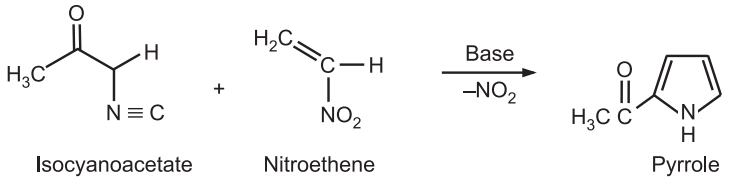
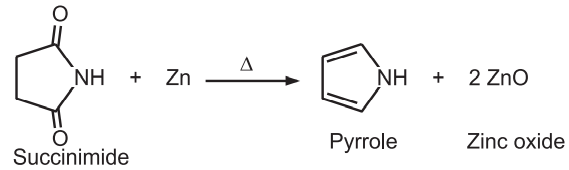
(6) From the distillation of succinimide: Pyrrole is obtained by the distillation of succinimide with zinc dust.
(7) From acetylene and ammonia: Pyrrole is obtained by passing acetylene and ammonia through a red hot tube.
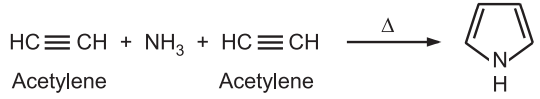
Chemical Reactions Of Pyrrole
Pyrrole, furan, and thiophene, all are π – excessive rings. Hence, all these rings are prone to electrophilic substitution reactions on the ring carbons. The order of reactivity for electrophile attack is pyrrole > furan > thiophene > benzene. The greater reactivity of pyrrole towards electrophiles is due to the greater electron releasing ability of the N – atom than oxygen and a sulfur atom. Thiophene is the least reactive.
These heterocycles are π – excessive rings. Hence, these heterocycles are less reactive towards nucleophilic substitution reactions except deprotonation at the N-atom or C-atom. Pyrrole behaves both as a weak base and a weak acid. e.g.,
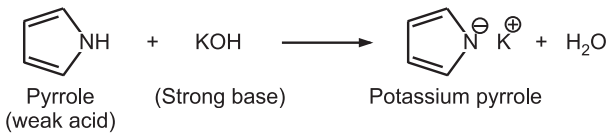
Hence, the proton attached to the nitrogen atom undergoes easy deprotonation in both acid and alkali. Similar deprotonation of carbon atoms in the ring occurs only in more acidic conditions. The α – protons (C2 & C5) exchange occurs at twice the rate of the β-protons (C3 & C4).
(1) Alkylation and arylation: The sodium/potassium salt of pyrrole reacts with an alkyl halide to give corresponding N – alkyl pyrrole. The presence of electron-withdrawing substituent on the pyrrole ring favors rapid N-alkylation or N-arylation.
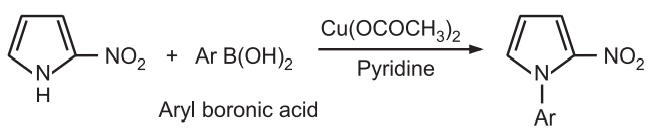
However mono C – alkylation of pyrrole can not be achieved by direct reaction with alkyl halides.
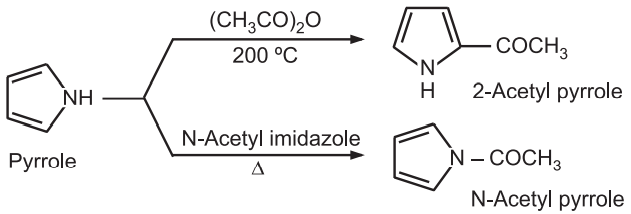
(2) Acylation: Pyrrole treated with acetic anhydride at 200°C gives 2 – acetyl pyrrole while N – acetyl pyrrole can be obtained by heating pyrrole with N – acetyl imidazole.
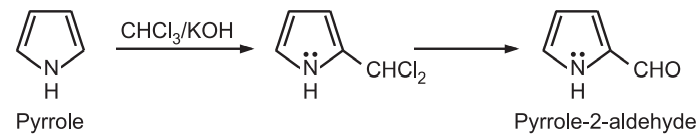
(3) Reimer – Tiemann reaction: In the presence of a strong base and chloroform, pyrrole undergoes Reimer – Tiemann reaction to form pyrrole – 2 – aldehyde.
(4) Ring expansion: When potassium pyrrole is heated with chloroform and sodium ethoxide, the pyrrole (five-membered ring) expands to pyridine (six-membered ring).
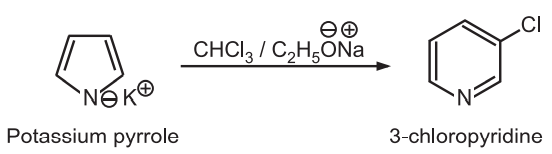
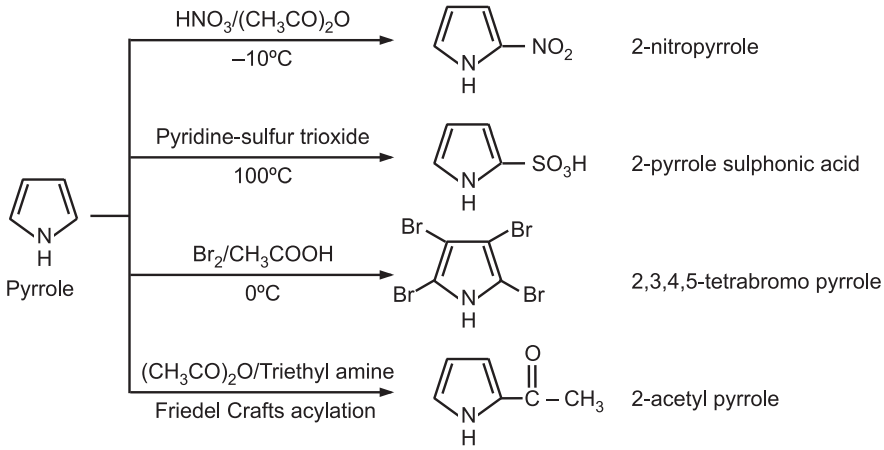
(5) Electrophilic substitution: The electrophilic substitution takes place preferably at C2 or C5 – positions. If these positions are already occupied then substitution takes place at C3 or C4 positions.
(6) Vilsmeier-Haack reaction: Pyrrole may be formylated by heating it with phosphorus oxychloride and dimethylformamide. The intermediate is hydrolyzed in the presence of a mild base to 2 – pyrrole carbaldehyde.
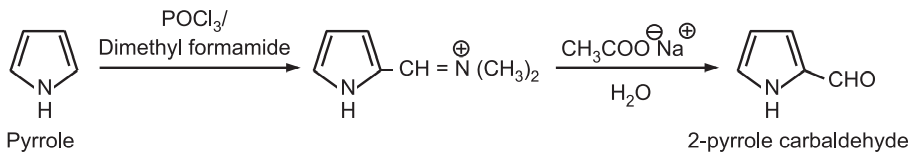
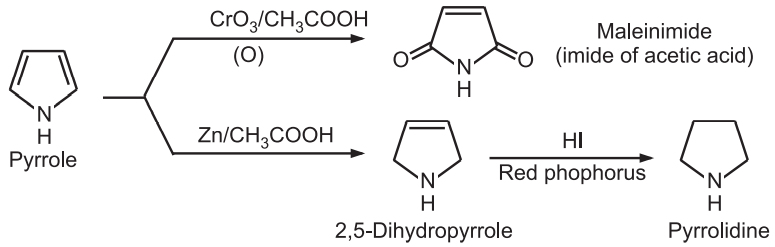
(7) Oxidation and reduction: Pyrrole is oxidized to maleimide and on reduction it gives pyrrolidine.
Applications in Drug Synthesis
Pyrrole is a structural constituent of haem, chlorophyll, Vitamin B12, and bile pigments. Pyrrole ring is also present in the drug tolmetin (NSAID), ketorolac (NSAID), sunitinib (anti-cancer), age life in (anti-bacterial), elopiprazole (antipsychotic), procyclidine (an antimuscarinic drug to treat parkinsonism), and atorvastatin (lipid-lowering agent). Pyrrole is widely known as a biologically active scaffold having diversified therapeutic activities such as antipsychotic, β-adrenergic antagonist, anxiolytic, antibacterial, antifungal, antimalarial, and anticancer.
Make sure you also check our other amazing Article on : Conformational Isomerism
