Chemical Synthesis and Reactions of Thiophene: Thiophene belongs to a class of heterocyclic compounds containing a five-membered ring made up of one sulfur as a heteroatom. Thiophene is a colorless liquid having a boiling point of 84°C. It was isolated as an impurity in commercial benzene in 1882 by Victor Meyer. It is a π – excessive aromatic heterocycle.
Chemical Synthesis of Thiophene
(1) Paal-Knorr Synthesis: In this method, 1, 4 – dicarbonyl compounds can be heated with phosphorus pentasulfide (a source of sulfur) to give thiophene.
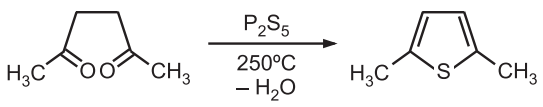
The basic mechanism of this synthetic procedure involves cyclic condensation of 1, 4-diketones (a) with primary amine (pyrrole synthesis), (b) with a sulfur source (thiophene synthesis), or (c) dehydration of diketone (furan synthesis). Phosphorus pentasulfide or bis (trimethylsilyl) sulfide acts as a sulfurizing agent as well as a dehydrating agent. Hydrogen sulfide in the presence of an acid catalyst is also effective.
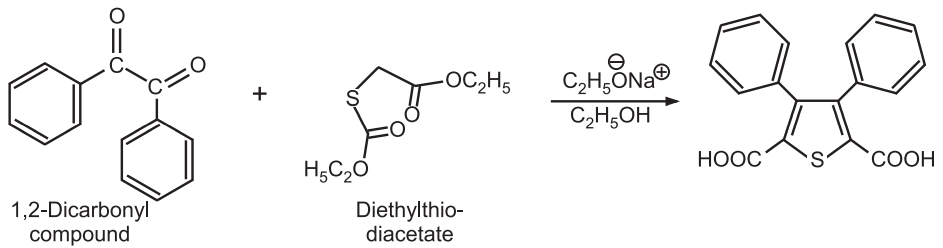
(2) Hinsberg Synthesis: Two consecutive aldol condensations between 1, 2-dicarbonyl compound and diethylthiodiacetate in the presence of a strong base gives thiophene.
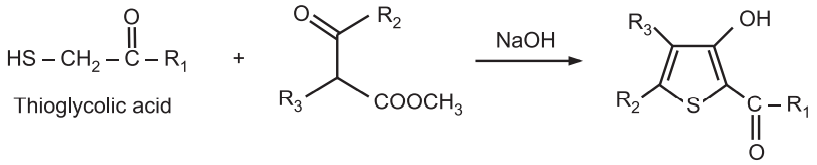
(3) Fiesselmann Thiophene Synthesis: It is a base-catalyzed condensation reaction of thioglycolic acid with α, β-acetylenic esters to give 3-hydroxy-2-thiophene carboxylic acid.
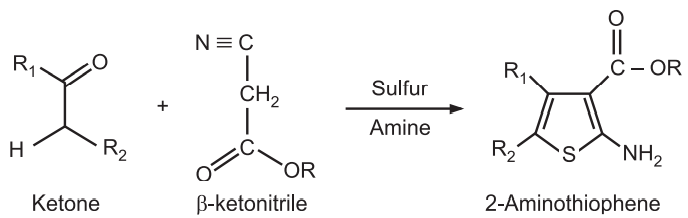
(4) Gewald Aminothiophene Synthesis: It is a base-catalyzed condensation of a ketone with a β–acetonitrile to form an olefin, followed by cyclization with elemental sulfur to give 2-aminothiophenes.
(5) Industrial Methods:
(i) Thiophene can be synthesized on an industrial scale heating n-butane and sulfur at high temperatures.
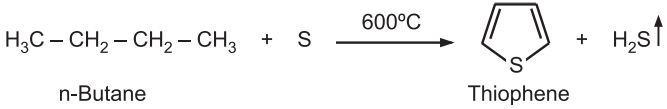
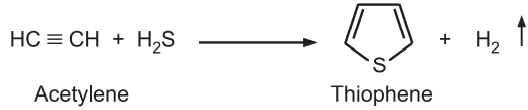
(ii) Using n-butane the sulfur first causes dehydrogenation and then interacts with the alkene by addition. Further dehydrogenation leads to the aromatization of the ring. A mixture of acetylene and hydrogen sulfide is passed through a tube containing alumina at 400°C.
(iii) A mixture of sodium succinate and phosphorus trisulfide is heated at 200°C to give thiophene.
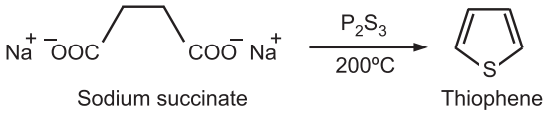
Chemical Reactions of Thiophene
Thiophene is slightly more nucleophilic than benzene. A negative charge is more accumulated on C2 – and C5 – atoms. While sulfur bears a positive charge. Hence, it easily undergoes electrophilic substitution preferably at C2 – and C5 – positions.
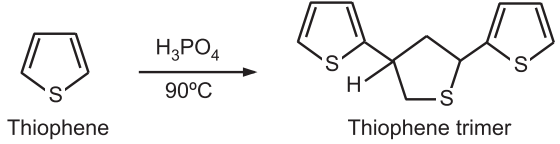
(1) Protonation: Thiophene is very stable to the action of acids. Very strong acids like the action of hot phosphoric acid give thiophene trimer.
(2) Oxidation: Thiophene ring is stable to the action of various oxidizing agents. However, the side chains can be oxidized to carboxylic acid groups. When heated with nitric acid, the ring breaks down to maleic acid and oxalic acid.
(3) Electrophilic Substitution: The reactivity order for electrophilic substitution reaction is: pyrrole > furan > thiophene >benzene. The preferred site of the attack in thiophene is the C2-position.
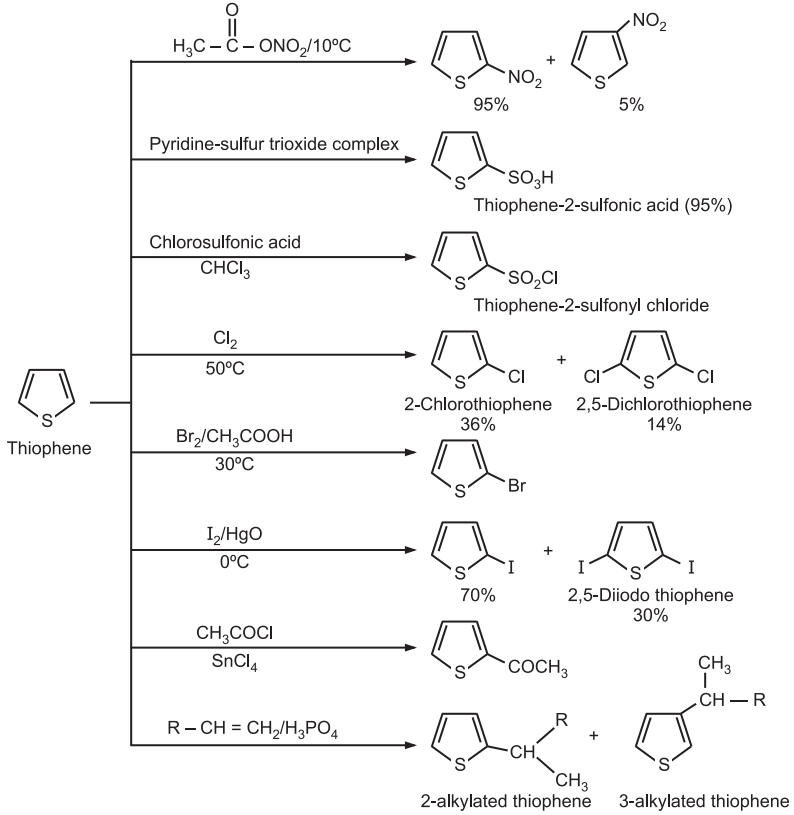
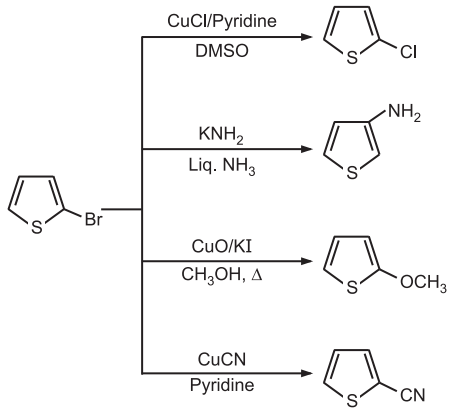
(4) Nucleophilic Substitution: Thiophene substituted with electron-withdrawing substituents are much more reactive to nucleophilic substitution.
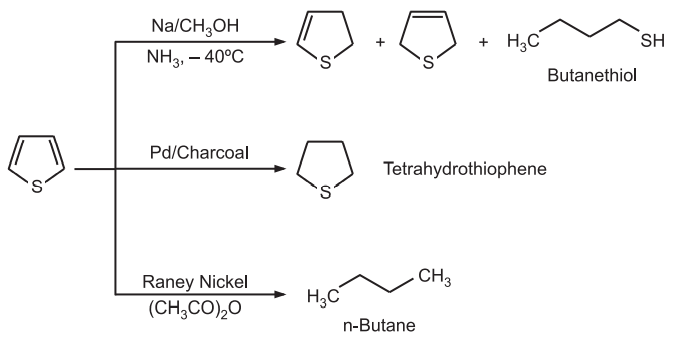
(5) Reduction:
Applications in Drug Synthesis
Thiophene derivatives possess remarkable activities like antibacterial, anti-inflammatory, anti-anxiety, anti-psychotic, anti-arrhythmic, and anticancer. Examples include lornoxicam (thiophene analog of piroxicam), pyrantel (anti-parasitic), raltitrexed (anticancer), cephalothin (antimicrobial), suproprofen (anti-inflammatory), ticrynafen (anti-hypertensive), clotiazepam (anti-anxiety), ticlopidine (platelet aggregation inhibitor), etc.
Make sure you also check our other amazing Article on : Chemical Synthesis and Reactions of Furan
