Classification of Cosmeceutical Products: Cosmetic science is a fast-moving area. Furthermore, rapid and extensive changes in the worldwide regulatory context of cosmetics, increasing constraints and limitations in the choice of cosmetic ingredients, and regular pressure from the media force the cosmetics formulator to think differently about his products. According to USFDA, the definition of cosmetics and drugs is clear and simple. However, there is one legal parameter that thoroughly confounds the issue, that cosmetics must “not affect structure and function of the skin.” This statutory differentiation of drugs from cosmetics was probably appropriate for the state of knowledge when the USFDA rules were written, more than a half-century ago. Since then there has been a tremendous increase in knowledge of the physiology of skin has brought the law and biology into conflict. The truth is that all topical substances, whether as simple as water or as complex as multi-ingredient moisturizers, inevitably will affect the structure and function of the skin. No topical product is completely inert. In 1938, the ideas regarding skin physiology were primitive. Now it is known that skin exposed to water for 48 hours demonstrates cytokine release, producing a condition known as hydration dermatitis. Under electron microscopy, water can produce changes in Langerhans cell and mast cell function. Hydration dermatitis is a disease, but water is not a drug. Since everything applied to the skin produces change, so there is a need for a third category of products known as cosmeceuticals. The concept of cosmeceuticals is comparatively new in India and not many rules and regulations exist to abide by them. Conceptually, they fall under the grey area of conventional drugs and cosmetics. In most countries, a suitable regulatory category for these hybrid products does not exist and therefore most complications in market development arise from a lack of a clear definition and the consequent legal framework for cosmeceuticals. Some cosmeceuticals are naturally-derived while others are synthetic; but all contain functional ingredients with either therapeutic, disease-fighting, or healing properties.
Mechanism of Action of Cosmeceuticals
Table of Contents
Cosmeceuticals improve appearance, but they do so by delivering nutrients necessary for healthy skin. The cosmeceutical products act functionally. Evidence to support the claims or use of cosmeceutical ingredients is often lacking in the literature. Many contain biologically active ingredients, and in general, cosmeceuticals undergo tests to determine safety, but claims of efficacy are largely unsubstantiated. Efforts have only recently been initiated to address the issues surrounding quality control and to establish industry standards and regulations. Demonstrating the skin effect of a cosmeceutical can be difficult; there are no placebos because anything that is applied to the skin will have an effect. However, ten basic mechanisms of action of cosmeceuticals are enlisted in Table.1 below.
Table.1: Ten basic mechanisms of action of cosmeceuticals
| Sr. No. | Mechanism of Action | Example of Cosmeceutical |
| 1. | Activate a receptor | Retinoids: Tretinoin, Retinol |
| 2. | Enhance barrier function | Moisturizers based on Petrolatum, Silicone, Mineral oil, Glycerin |
| 3. | Increase exfoliation | Salicylic acid |
| 4. | Normalize cellular repair | Copper peptides |
| 5. | Decrease inflammation | Green tea |
| 6. | Inhibit oxidation | Lactobionic acid, Vitamin E |
| 7. | Provide a cellular messenger | Pentapeptides |
| 8. | Regulate cellular communication | Hexapeptides |
| 9. | Modulate pigmentation | Avobenzone, micronized titanium dioxide |
| 10. | Deliver photo-protection | Avobenzone, micronized titanium dioxide, and microfine zinc oxide |
Classification of Cosmeceutical Products
The cosmeceuticals are divided into eight categories as shown in Fig.1 below.
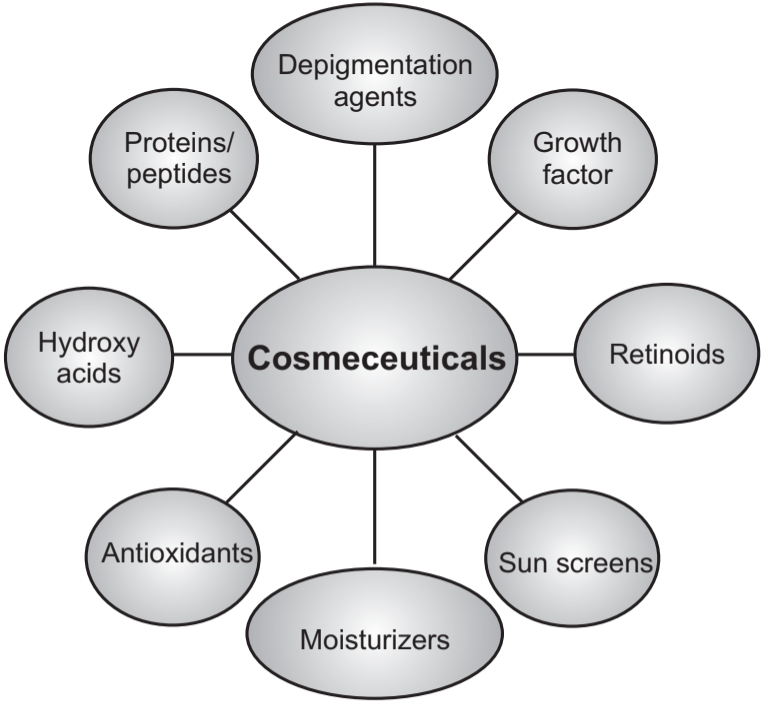
1. Retinoids
Retinoids are premier evidence-based cosmeceuticals, as they function through surface cell receptor interaction to produce a clinically defined effect. Other retinoids such as pro-B vitamins (niacinamide and panthenol) function differently by physically enhancing barrier properties of the stratum corneum. These are the most prevalent cosmeceuticals in the market. They consist of natural and synthetic derivatives of vitamin A that reduce hyperpigmentation and inhibit enzymes from breaking down collagen. Many of their cosmeceutical claims are based on data derived from studies on tretinoin and other classes of retinoid drugs. Some key retinoids include retinoic acid (tretinoin), retinol, retinaldehyde.
Retinoic Acid (Tretinoin): There is extensive literature on the use of tretinoin, which is considered to be one of the most potent compounds for treating the signs of aging and/or photodamaged skin, including fine lines, hyperpigmented spots, and wrinkles. However, side effects such as burning and scaling have limited its acceptance. To minimize these side effects, various novel drug delivery systems are being developed.
Retinol (Vitamin A): Retinol is oxidized into retinaldehyde and then into retinoic acid, the biologically active form of vitamin A. In vivo studies showed that topical retinol had only a modest retinoid-like biological activity compared with topical retinaldehyde and retinoic acid. Two randomized, controlled trials reported significant improvement in fine wrinkles after 12 and 24 weeks of treatment, respectively.
Retinaldehyde: Retinaldehyde is viewed in a large part as an intermediate form during the conversion of retinol to retinoic acid. Studies have shown that it does have activity in human skin. Moreover, some studies have reported that this retinoid can produce significant clinical improvement in the appearance of fine and deep wrinkles.
2. Sunscreens
Sunscreens are the single most important cosmeceutical because they protect skin against solar radiation, which is the most important damaging environmental agent. As a result, they help to prevent the signs of aging. To be effective, sunscreens should provide broad-spectrum coverage that includes both UVA and UVB blocking agents to inhibit photoaging and be part of a daily skincare regimen. UVA and UVB radiation contribute to the disruption of the extracellular matrix, a vital phenomenon related to photoaging. Broadspectrum UVA and UVB sunscreens are the cornerstone of photoaging therapy. Sunscreens contain active ingredients that act as ultraviolet filters. Enzophenones (dioxybenzone, oxybenzone, sulisobenzone) give protection in the UVB and UVA II range (320–340 nm). The recommended application is 2mg/cm2, though this is rarely achieved in real-life practice.
Green Tea Extract: Research has shown that green tea (Camellia Sinensis) polyphenols are potent suppressors of carcinogenic activity from UV radiation and can exert broad protection against other UV-mediated responses, such as sunburn, immunosuppression, and photoaging.
Ferulic Acid: This compound, derived from plants, is considered to be a potent antioxidant, and has been shown to provide photoprotection to skin. Furthermore, when ferulic acid is combined with vitamins C and E, the product has been shown to provide substantial UV protection for human skin. Moreover, because its mechanism of action is different from sunscreens, ferulic acid could be expected to supplement the sun protection provided by sunscreens.
3. Moisturizers
Moisturizers are the most useful product for the management of various skin conditions (e.g., atopic dermatitis, psoriasis, pruritus, and aging skin). These products include emollients, occlusives, and humectants. The majority of moisturizers enhance skin barrier function. Moisturizers claim to make the skin smoother, softer, more radiant, less wrinkled, and firmer. They improve the tactile properties of dry and aging skin, restore the normal barrier function of the skin, and reduce the release of inflammatory cytokines. Moisturizers based on materials such as petrolatum, silicon, mineral oil, and glycerin enhance skin barrier functions. Moisturizers restore water content to the epidermis and provide a soothing protective film.
4. Antioxidants
Topically applied antioxidants enhance the skin’s natural antioxidant protection system. They reduce free-radical damage by blocking the oxidative processes in cells. These are used to protect skin from photodamage, cancer, and photoaging. Antioxidants inhibit inflammation that causes collagen depletion. They protect against photodamage and skin cancer. However, there is no completely satisfactory agent available for humans. Explanations for this could include the fact that:
- Reactive oxygen species (ROS) affect different pathways in different situations and an antioxidant focused on one such pathway may be ineffective in a redundant pathway.
- ROS pharmacokinetics in the target tissue may not relate to that of the antioxidant.
- Bioavailability and target organ concentration of the antioxidant may be a limiting issue.
Common antioxidants include alpha-lipoic acid (ALA), L-ascorbic acid (vitamin C), niacinamide (vitamin B3), N-acetyl-glucosamine (NAG), á-tocopherol, and ubiquinone (CoQ10) that are described below.
Alpha-Lipoic Acid (ALA): Alpha-lipoic acid has anti-inflammatory properties and acts as an exfoliant. In a split-face study, topical 5% ALA applied b.i.d. for 12 weeks reduced skin roughness, lentigines, and fine wrinkles. This agent does not protect against UV-induced erythema or reduce the number of sunburn cells.
L-Ascorbic Acid (Vitamin C): There is clinical data to support the use of topical vitamin C to improve fine lines and reduce both pigmentation and inflammation, and many cosmeceutical formulations contain this antioxidant. However, many of these formulations are not effective on the skin because:
- The concentration of L-ascorbic acid is too low.
- Exposure of the product to air and light compromises the stability of the product.
- The L-ascorbic acid molecule (in the form of an ester or a mixture of isomers) cannot be absorbed or metabolized effectively by the skin.
In high enough concentrations (i.e., at least 10%) of the non-esterified, optimal isomer, this antioxidant does inhibit UV damage. It is important to note that stabilizing ascorbic acid presents many formulary challenges. However, a formulation that has an acid pH of approximately 3, may optimize vitamin C absorption. Newer formulations of stabilized ascorbic acid derivatives may prove to be more efficacious.
Niacinamide (Vitamin B3): Niacinamide is a potent antioxidant that is generally well tolerated. It improves the lipid barrier component of the epidermis, thus reducing transepidermal water loss, and acts as an inhibitor of melanosome transfer, resulting in reduced hyperpigmentation. Studies have revealed a significant reduction in fine lines and wrinkles, hyperpigmented spots, red blotchiness, and skin sallowness, as well as improved skin elasticity.
N-Acetyl-Glucosamine (NAG): NAG is a more stable form of glucosamine, and may prevent new signs of photodamage from occurring, and fade existing imperfections by interrupting the chemical signals that promote melanin production. A placebo-controlled study comparing 3.5% NAG with the combination of 3.5% NAG plus 3.5% niacinamide on hyperpigmented spots showed a superior reduction in pigmentation in the combination treatment group versus both the placebo and NAG only groups. When combined, they produce synergistic effects.
Alpha-Tocopherol (Vitamin E): When taken orally, α-tocopherol protects membrane lipids from peroxidation. It has been shown to reduce sunburn cells after UV exposure, neutralize free radicals, and act as a humectant. Its activity can be renewed by combining it with vitamin C preparation. As a component in topical formulations, it, like unmodified L-ascorbic acid, has shown some limited efficacy. However, when a stable formulation delivers a high concentration of the non-esterified, optimal isomer of this antioxidant, vitamin E does inhibit the acute UV damage of erythema, sunburn, and tanning, as well as chronic UV photoaging and skin cancer. Because vitamin C regenerates oxidized vitamin E, the combination in a cosmeceutical formulation is synergistic – particularly about UV protection.
Ubiquinone (CoQ10): Ubiquinone is a naturally occurring, fat-soluble antioxidant and there is good in vitro evidence that it can suppress fibroblast production of UVA-induced collagenase, thereby reducing collagen breakdown. It is effective against UVA-mediated oxidative stress in human keratinocytes. Ubiquinone was also able to significantly suppress the expression of collagenase in human dermal fibroblasts following UVA irradiation. Another study showed that ubiquinone can strongly inhibit oxidative stress in the skin induced by UVB. It is an effective antioxidant protecting the dermal matrix from both intrinsic and extrinsic aging.
Grape Seed Extract: It is a potent antioxidant and has been shown to speed wound contraction and closure. Topical application of grape seed extract has also been shown to enhance the sun protection factor in humans.
5. Hydroxy acids
These include α-hydroxy acids (AHAs; glycolic acid, lactic acid) and β-hydroxy acids (BHAs; salicylic acid). Hydroxy acids are used worldwide and most probably for centuries as active dermatological drugs and cosmetic ingredients. The exact mechanism of action of hydroxy acids remains unknown and is largely controversial. Some experts claim that AHAs increase the synthesis of glycosaminoglycans which improve the quality of elastic fibers, and increase the density of collagen; whereas BHAs have hemolytic properties and help in various xerotic and ichthyotic disorders. AHAs are also referred to as fruit acids and are a common ingredient of cosmeceutical products. Examples include citric acid, malic acid, glycolic acids, pyruvic acid, lactic acid, tartaric acid. AHAs improve skin texture and reduce the signs of aging by promoting cell shedding in the outer layers of the epidermis and by restoring hydration. The mechanism of action is not completely understood. One hypothesis suggests that AHAs reduce the calcium ion concentration in the epidermis and, through chelation, remove the ions from the cell adhesions, which are thereby disrupted, resulting in desquamation. This is enhanced by cleavage of the endogenous stratum corneum chymotryptic enzyme on the cadherins, which are otherwise protected from proteolysis by conjugation with calcium ions. The resulting reduction of the calcium ion levels tends to promote cell growth and slow cell differentiation, thus giving rise to younger-looking skin.
6. Topical Proteins and Peptides
Cosmeceutical peptides have the potential to improve the appearance of aging skin. Topical peptides are regarded as cellular messengers that are formed from amino acids and are designed to mimic peptide fragments with endogenous biologic activity. These pentapeptides (e.g., KTTKS) are comprised of a subfragment of type I collagen propeptide, and play a role in signaling fibroblasts to produce collagen in the skin, which can improve the appearance of wrinkles. One variation, the palmitoyl pentapeptide known as Pal-KKTKS (Matrixyl™, Sederma) was tested in a controlled, double-blind, left-right randomized, split-face study of 93 women between 35 and 55 years of age who had Fitzpatrick I-III type skin. Pal-KTTKS concentration was 3 ppm; both groups were treated twice daily for 12 weeks. Improvements in wrinkle appearance and length were observed.
There are various types of cosmeceutical peptides such as signal peptides, carrier peptides, and neurotransmitter inhibiting peptides. Overall cosmeceutical peptides trigger a wound-healing mechanism that activates fibroblasts in response to fragmented chains of elastin and collagen. Peptides increase collagen production to improve skin appearance, resulting in smoother skin.
7. Depigmentation agents
Skin-lightening agents added to product formulations have become increasingly popular. Common depigmenting ingredients include hydroquinone, ascorbic acid (vitamin C), kojic acid, and licorice extract (glabridin).
Hydroquinone: Hydroquinone has been the agent of choice for skin lightening. However, there are concerns over exogenous ochronosis and permanent depigmentation, as well as possible carcinogenicity, and it has been banned as an over-the-counter depigmenting agent in Europe, Australia, and Japan. The US FDA has proposed concentrations between 1.5% and 2% in skin lighteners. A recent report suggested that this concern has been based mainly on studies with animal models utilizing long-term exposure at high dosages. Routine topical application may pose no greater risk than that from levels present in common foods. Hydroquinone is effective and widely used for the treatment of melasma, post-inflammatory hyperpigmentation. It acts by inhibiting the conversion of tyrosine to melanin.
Ascorbic acid (Vitamin C): Ascorbic acid is a naturally occurring antioxidant found in citrus fruits and leafy green vegetables. It is hydrophilic, so skin penetration is low.
Kojic acid: Kojic acid is a less commonly used bleaching agent. When combined with dipalmitate, there is improved skin penetration and greater stability, but there is little research to support its efficacy.
Licorice Extract (Glabridin): Several studies on melasma have shown good efficacy with only mild irritation that disappeared with discontinuation.
8. Growth factors
Epidermal growth factor (EGF) stimulates epidermal growth and is used in the treatment of burns and excision wounds, where it accelerates re-epithelization. Transforming growth factor (TGF) stimulates normal skin growth and cellular growth and repair. TGF exerts positive regulatory effects on the accumulation of the body’s extracellular matrix proteins.
TGF is also a mediator of fibrosis (repair tissue formation) and angiogenesis (development of new blood cells) and it promotes the healing of wounds.
Formulation Considerations
Although some products claim for the active ingredients used in cosmeceutical formulations are evidence-based, consumers often place their confidence in the claims made by the manufacturer. Without testing to assess the efficacy of key active ingredients about overall product content, it is possible that at inadequate concentrations, any beneficial effect will become unapparent. Ensuring consistency of formulations is also an area that has been neglected and necessitates regulation.
One of the most important parts of any cosmeceutical is the vehicle that carries the active ingredient into the skin. Vehicle delivery systems can:
- Augment the efficacy of the active ingredient
- Inactivate the active ingredient
- Improve the skin barrier
- Provoke allergic contact dermatitis.
In some skin conditions, the vehicle may be as good as the active preparation, and it may take three months or more to see a difference.
Regulating Cosmeceuticals
Cosmetic and drug regulations did not exist in the early 20th century. Probably at that time cosmeceutical term itself was non-existing. A few tragic incidents triggered the government authorities to put forward regulations about the safety and efficacy of medicines. Then the regulations concerning cosmetics and some advanced products were framed. The majority of the countries followed a similar path about regulations of medicines, cosmetics, medical devices, and some advanced products like cosmeceuticals, nutraceuticals, etc. Cosmeceutical products are borderline products having the attributes of both cosmetic and medicine. Hence, regulating cosmeceuticals is an area of interest. There are major differences among regulatory authorities regarding cosmeceuticals, starting from the term to the various regulatory requirements. A regulatory system for cosmeceuticals is said to be at the evolution stage, where deliberations are on among various stakeholders of the cosmeceutical industry. Regulatory agencies around the globe have not yet formally recognized cosmeceuticals as a separate product category, despite a rapid proliferation of cosmetic products that have a documented and intended pharmaceutical activity.
The current regulatory system in India does not adequately assure continued access to the safety of cosmeceuticals. By long-standing statute, the world of topical is divided into two opposed groups, drug versus cosmetics. According to the Drugs and Cosmetics Act, 1940, Drug is “all medicines for internal or external use of human beings or animals and all substances intended to be used for; or in the diagnosis, treatment, mitigation or prevention of any disease or disorder in human beings or animals.” Cosmetic is defined as “any article intended to be rubbed, poured, sprinkled or sprayed on or introduced into or applied to any part of the human body, for cleansing, beautifying, promoting attractiveness or altering the appearance and includes any article intended for use as a component of cosmetic”.
According to the definition of drug and cosmetic, these products (cosmeceuticals) do not fit precisely into either the current “drug” or “cosmetic” category. And while cosmeceuticals may be marketed under OTC or cosmetic category which provides a regulatory framework, these may not be sold as medicines. Thus, a present trouble is that there is no sharp dividing line between these categories. The current legal definitions of drugs and cosmetics are archaic and unworkable, as indeed formally noted by the dermatology community nearly 20 years ago.
The transition towards the acceptability of this new trend of cosmeceuticals is relatively slow as far as regulatory issues are concerned. The absence of a proper regulatory framework and dilemma over the understanding of the term “Cosmeceuticals” has become an undue advantage for cosmetic manufacturers. Manufacturing, labeling, sale, and advertisement of such products need a proper regulatory framework to safeguard the interest of consumers and control the cosmeceutical market. There is an urgent need to frame guidelines to regulate the manufacturing, labeling, and advertisement of cosmeceuticals in India. The regulatory framework may help:
- Various stakeholders in the field of cosmeceuticals.
- Support use of cosmeceuticals by health care professionals.
- Ensure and provide factual information to the public regarding cosmeceuticals.
- Shall protect the public from unproven claims and unsafe products.
- Shall drive growth and exports of cosmeceuticals.
Categorization of Cosmeceuticals in Few International Markets
Japan
Japan accommodates cosmeceuticals by calling them “quasi-drugs”. These are products that exert mild actions on the human body. The ingredients included in the quasi-drug must be pre-approved before being marketed in Japan. All products claiming to be cosmeceuticals are considered quasi-drugs and require pre-approval before selling in the market.
New Zealand
New Zealand law provides a third category called “related products”. Related products are those having therapeutic use as a purpose secondary to the main use. Related products are to be labeled with an appropriate designation and trade name, the active ingredients to be disclosed quantitatively, the product’s true nature, expiry date and batch number, a dose and its frequency, directions for use, and name and address of the manufacture.
Korea
Korea Food and Drug Administration (KFDA) has classified cosmeceuticals or borderline products as “functional cosmetics” such as skin whitening, anti-aging, and sun care products. Functional cosmetics prevent melanin pigmentation, spots, promote whitening of the skin, improve skin wrinkles, and block or diffuse UV rays to protect the skin. The KFDA is responsible for evaluating and improving the safety of functional cosmetics.
Thailand
Under the current cosmetic regulation, cosmetics are classified as “controlled cosmetics” according to the ingredients used. The use of controlled ingredients as part of cosmetic products will require the notification of the products to FDA before being marketed in Thailand.
Australia
In Australia the categorization of goods is based on two factors: (a) Claims made about the product and (b) The composition of the product. The products which are at the borderline are classified as therapeutic goods. These goods must use only approved ingredients. The goods must be included in the Australian Register of Therapeutic Goods. Safety and efficacy, and Good Manufacturing Practices data must be submitted to a regulatory authority. The National Coordinating Committee on Therapeutic Goods (NCCTG) guides acceptable and unacceptable cosmetic claims.
Canada
In Canada cosmeceuticals are also called “dermo-cosmetics”. Canadian health authorities do not officially recognize cosmeceuticals as an independent cosmetics category. As several products fall into both categories of cosmetic and drug, Health Canada has identified category IV to accommodate these products. These products have fewer regulatory requirements because they have a low risk. The two key factors that are considered in the classification of a cosmetic versus drug are: the composition of the product and the proposed use of a product. Advertising Standards Canada, the Canadian Cosmetic, Toiletry and Fragrance Association, and the cosmetics division of Health Canada jointly have established the guidelines for cosmetic advertising and labeling claims. These guidelines help cosmetic manufacturers to use the wording of a claim on cosmetic products.
European Union
Cosmetics in the EU are regulated under the Cosmetic Directive 76/768/EEC. To avoid the categorization of cosmeceuticals, the EU has clarified by establishing the “Illustrative list by category of Cosmetic Products”. EU has stringent laws where companies are required to submit proof of the claims made by the product. As borderline products are already classified as cosmetics, The EU doesn’t need a third category called cosmeceuticals.
United States of America
According to USFDA, there is no legal definition of cosmeceutical products. In the US, there are three categories such as drugs, cosmetics, and OTC drugs. USFDA states that a product can be both drug and cosmetic. USFDA classifies products depending upon the product claim. In the US, the classification of products is neat and simple. Some of the examples are:
- A suntan product is cosmetic, but a sunscreen product is a drug.
- A deodorant is cosmetic, but antiperspirant is a drug.
- A skin exfoliant is cosmetic, but a skin peel is a drug.
- A skin product to hide acne is cosmetic, but an anti-acne product is a drug.
- A skin moisturizer is cosmetic, but a wrinkle remover is a drug.
- An antibacterial deodorant soap is cosmetic, but an antibacterial anti-infective soap is a drug.
- A lip softener is cosmetic, but a product for chapped lips is a drug.
- A shampoo is cosmetic, but an antidandruff shampoo is a drug.
- Toothpaste is a cosmetic, but anti-caries toothpaste is a drug.
- A mouthwash is cosmetic, but an anti-gingivitis mouth wash is a drug.
The categorizations of borderline or similar products are discussed in Table.2 with some examples in major markets.
Table.2: Categorization of borderline products with some examples
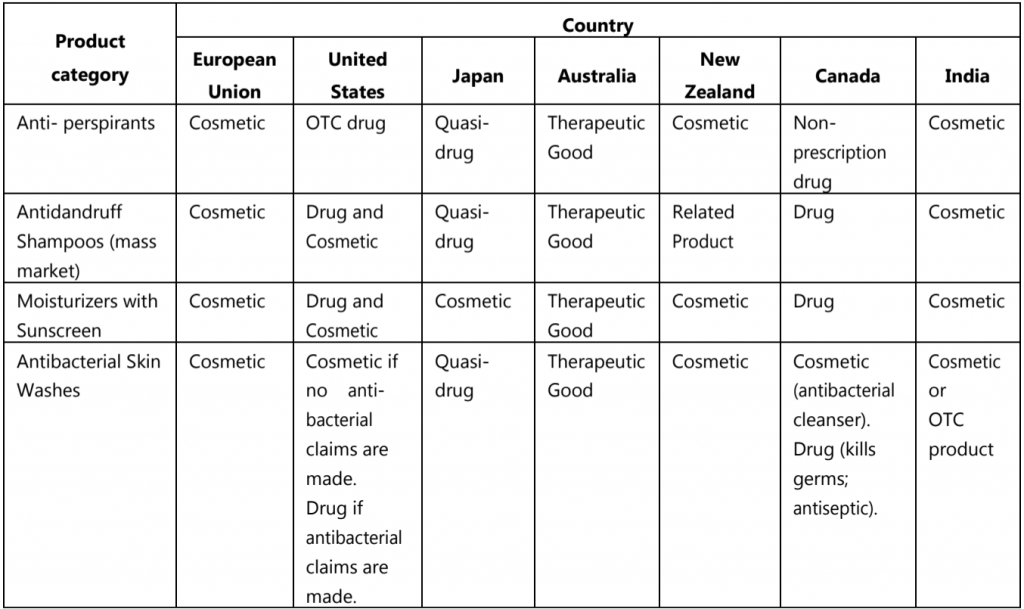
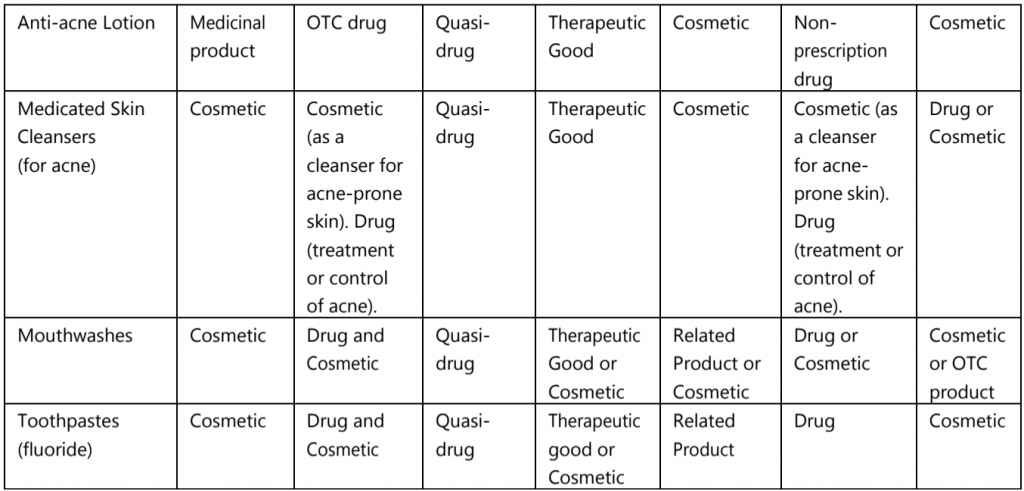
The borderline products are cosmetic in one market; the same product is OTC drug, non-prescription drug, or medicinal product in another market.
Make sure you also check our other amazing Article on : Cosmetics As OTC Drugs
