Classification of Flavonoids: Flavonoids are polyphenolic compounds and are vastly available in maximum plant species. They are generally yellow-coloured pigments. They are a larger group of the glycoside. They are 2-phenylbenzopyrones derivatives and produce a large number of physiological activities. Flavonoids are the largest group of naturally occurring phenols and occur in free states in the plants as glycosides. They may be described as a series of C6-C3-C6 compounds (Fig.1). They are largely found in Polygonaceae, Rutaceae, Fabaceae, and Rosaceae families.
Physical Properties:
- They are crystalline substances with a certain melting point.
- Catechins, Flavones, Isoflavanes, Flavanones, Flavanonoles are colourless crystals whereas Flavones, Flavonols, Chalcones are yellow coloured crystals. Anthocyanidins are red in acidic media and blue in alkaline media.
- Anthocyanins are sap pigments. The actual colour of the plant organ is determined by the pH of the sap. Example: The blue colour of the cornflower and the red colour of roses are due to these glycosides.
- Flavonoid glycosides are generally soluble in water and alcohol but insoluble in organic solvents.
- Aglycone parts of flavonoids are soluble in diethyl ether, acetone, alcohol etc.
- Flavanols are optically active.
- Flavanones and flavanones are unstable compounds.
- Flavonoid O-glycosides are undergoing hydrolysis when treated with acid, alkali.
- Rutin is yellow crystalline powder, soluble in alkali but slightly soluble in water.
- Rutin on hydrolysis gives quercetin, rhamnose and glucose whereas hesperidin yields hesperetin, rhamnose and glucose.
- Under the UV light flavonoids show fluorescence of different colours (yellow, orange, brown, red).
Chemical Properties:
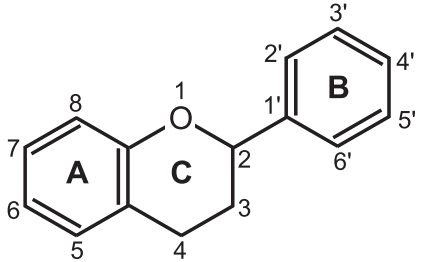
- Chemically flavonoids are based upon a fifteen-carbon skeleton (C15) consisting of two benzene rings (A and B) linked via a heterocyclic pyrene ring (C).
- They occur as aglycones, glycosides, and methylated derivatives.
- The Six-member ring condensed with the benzene ring is either an α-pyrone (flavonols and flavanones) or its dihydro derivative (flavonols and flavanones).
- The position of the benzenoid substituent divides the flavonoid class into flavonoids (2-position) and isoflavonoids (3-position).
- Flavonols differ from flavanones by hydroxyl group at the 3-position and a C2–C3 double bond.
- Flavonoids are often hydroxylated at positions 3, 5, 7, 2, 3′, 4′, and 5′. Methyl ethers and acetyl esters of the alcohol group are known to occur in nature.
- The glycosidic linkage is normally located in flavonoids at positions 3 or 7.
Classification of Flavonoids
Table of Contents
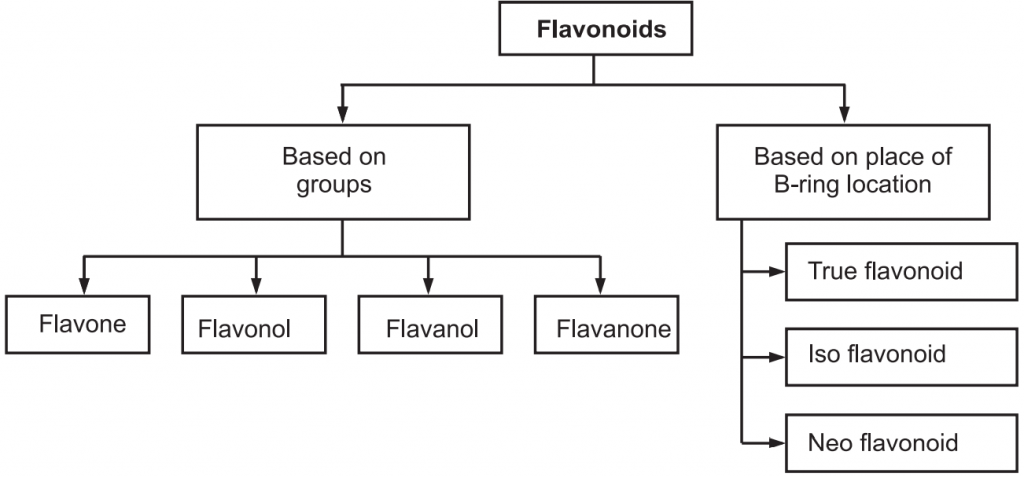
Broadly flavonoids are classified into two classes namely based on groups and based on place of B-ring location (Fig.2).
1. Based on Groups:
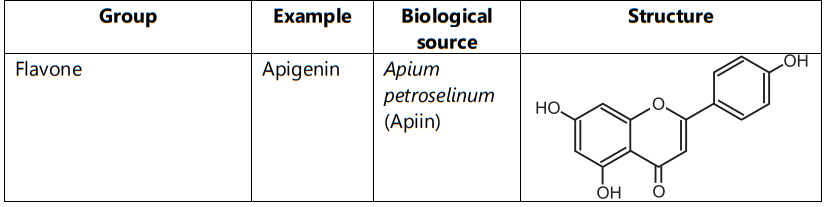
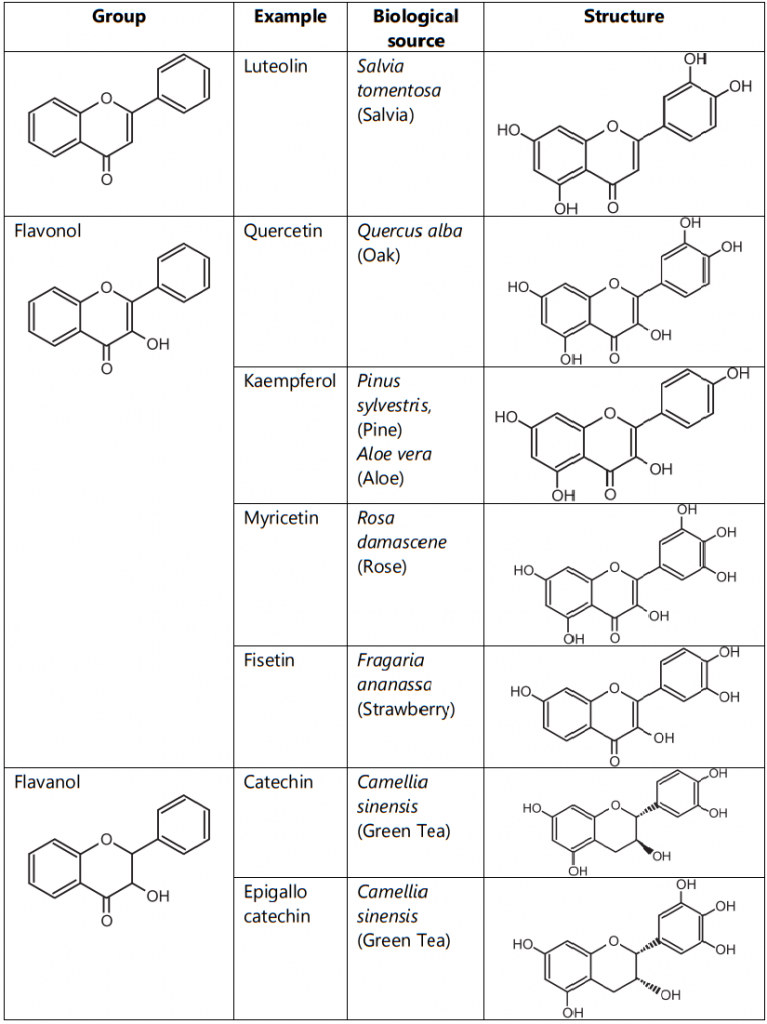
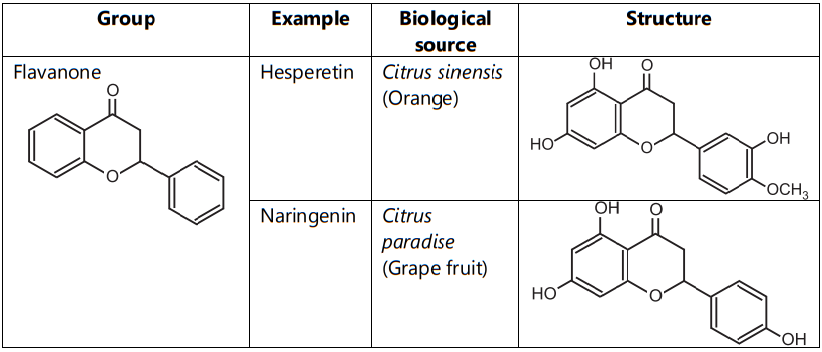
Flavonoids are classified into flavones (e.g. apigenin, and luteolin), flavonols (e.g. quercetin, kaempferol, myricetin, and fisetin), flavanones (e.g. flavanone, hesperetin, and naringenin), Flavanol (Example: Catechin) (Table.1)
Table.1: Classification of Flavonoids as per group
2. As per the Place of B-ring location:
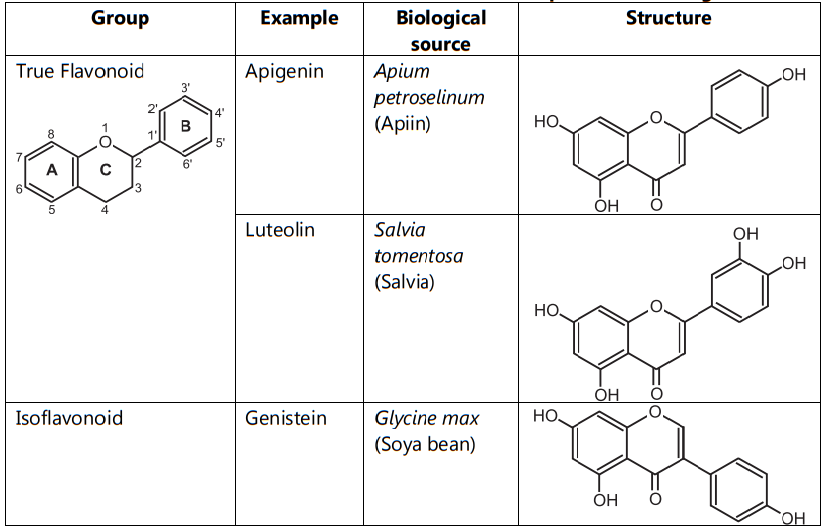
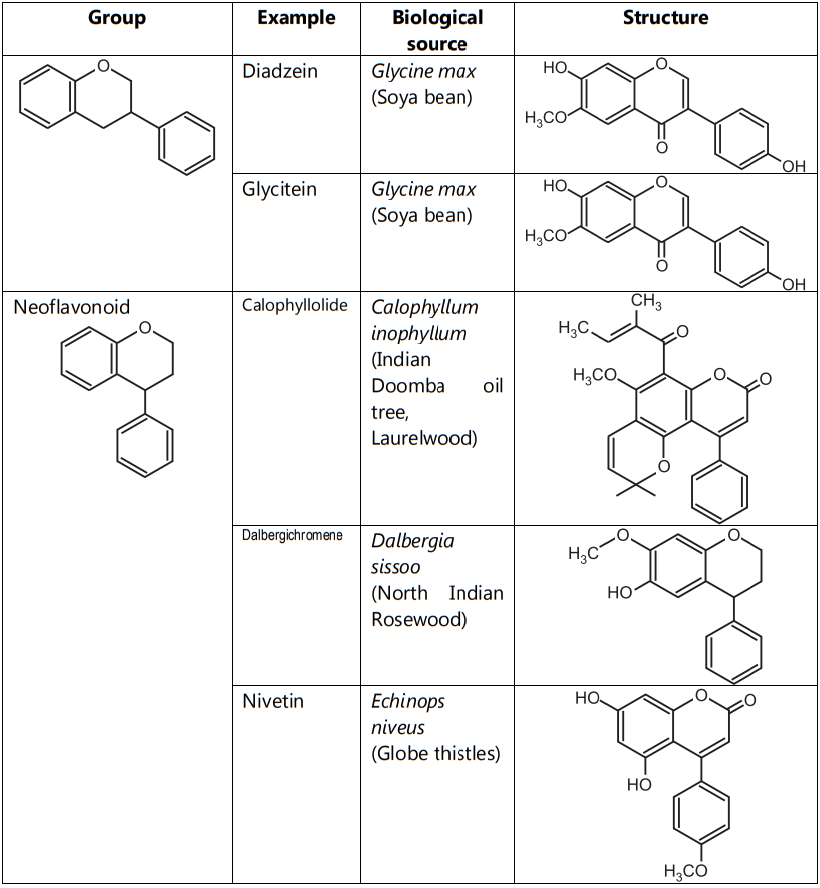
As per the location of the B-ring, they are three types like true flavonoids, isoflavonoid and neoflavonoid. True Flavonoids are derived from a 2-phenylchromen-4-one (2-phenyl-l, 4-benzopyrone) structure. Isoflavonoids are derived from 3-phenylchromen- 4-one (3-phenyl-1,4-benzopyrone) structure and Neo-flavonoids are derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure. True Flavonoids are also known as bioflavonoid due to origin from plants. Bioflavonoids are anthoxanthin i.e. mixture of flavones and flavonols (Table.2).
Table.2: Classification of flavonoids based on the position of B-ring
General Extraction:
Dried plant materials are powdered and extracted with various solvents based on the type of flavonoids such as less polar flavonoids (like Isoflavones, flavanones, flavonols) are extracted with chloroform, diethyl ether or ethyl acetate whereas more polar flavonoid glycosides are extracted with alcohol or a mixed solvent of alcohol and water. Soxhlet method is used for the extraction. An n-hexane solvent is used to defatted the plant materials followed by ethyl acetate solvent is used for the extraction of flavonoids.
Generally, a successive solvent extraction method is used for the extraction. First, dichloromethane is used for the extraction of flavonoid aglycone and other less polar constituents. Then alcohol is used for extraction of glycone part of flavonoid and other polar constituents. The crude extract is purified by using shaking with sodium carbonate or sodium bicarbonate (for strong acidic OH group) or by column chromatography using polyamide column, Sephadex LH-20 column. Sephadex LH-20 column is suitable for separation of proanthocyanidins where alcohol is used as an eluent and acetone is used for separation of high molecular weight polyphenols.
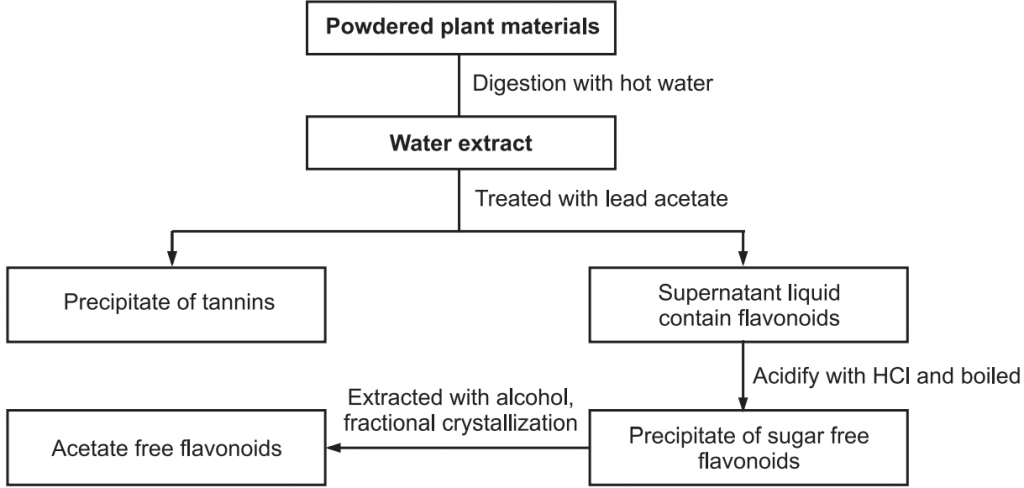
The easy method for common flavonoids extraction is described in Fig.3.
Chemical Tests for Flavonoid:
1. Shinoda Test: The alcoholic solution of flavone or flavonol when treated with metallic magnesium (or Zinc) and hydrochloric acid gives an orange, red or violet colour. This test is also known as the cyanidin reaction.
2. Lead Subacetate Test: Too small quantity of residue, add lead subacetate solution. The yellow coloured precipitate is formed. The addition of an increasing amount of sodium hydroxide to the residue shows yellow colouration, which decolouration after the addition of acid.
3. Wilson’s Reaction: Flavonoids form complexes with boric acid which is not destroyed by the addition of citric acid alcoholic solution (or oxalic acid).
4. Antimony Pentachloride Test: Alcoholic solution of the sample when reacts with antimony pentachloride the solution produces red or violet colour.
Functions:
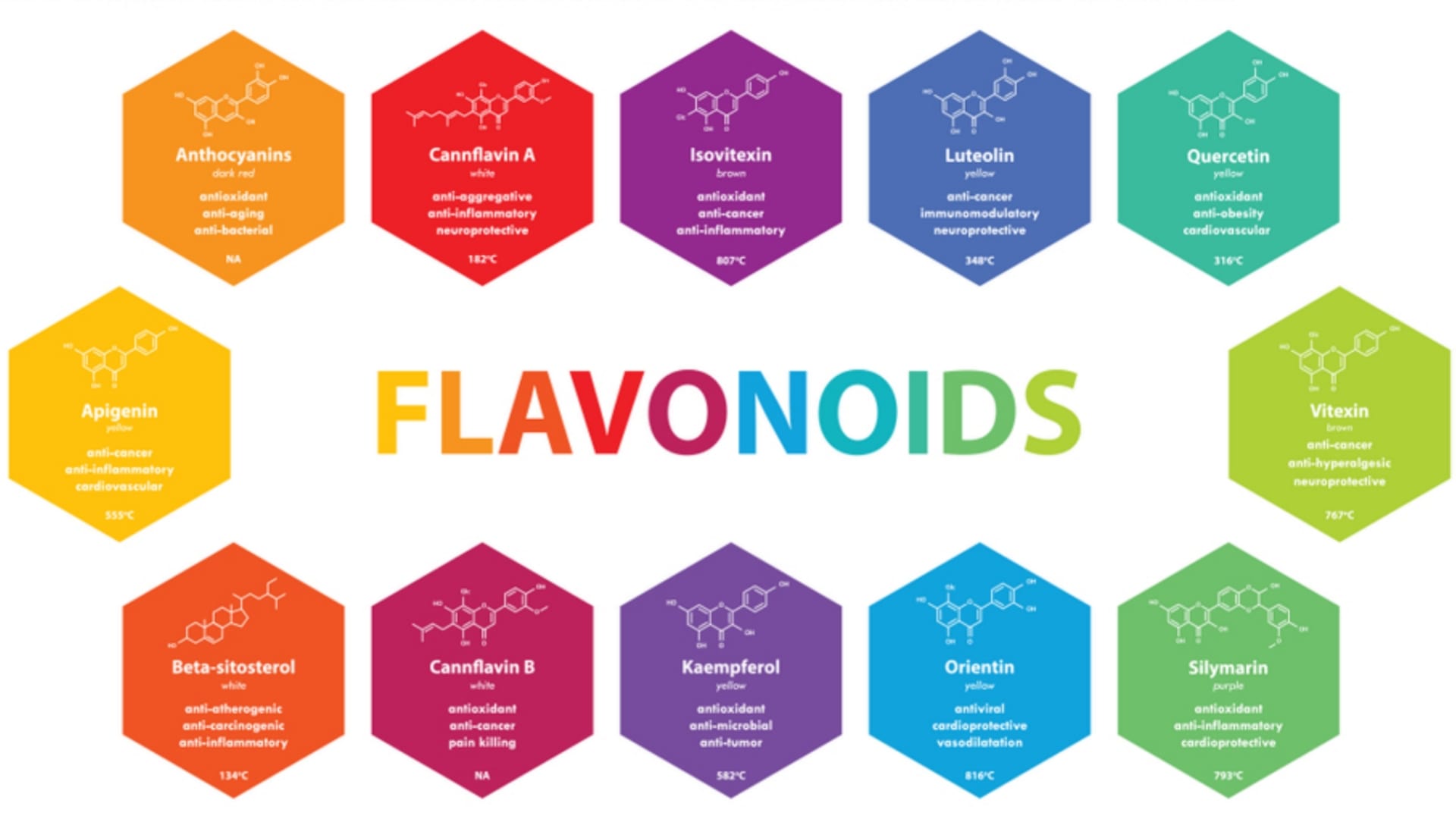
- They act as a powerful antioxidants like Quercetin, Xanthohumol, Isoxanthohumol etc.
- They control plant growth.
- They inhibit and activate plant enzymes.
- They having a role in the biochemistry of reproduction.
- They have fungicidal properties.
- They protect the plant from parasites attack.
- They are the pigments of flowers that attract insects for pollination.
- They are having significant therapeutic efficacy such as antiviral, antiallergic, antiplatelets, anti-inflammatory, antitumor etc.
Make sure you also check our other amazing Article on : Extraction Method of Alkaloids