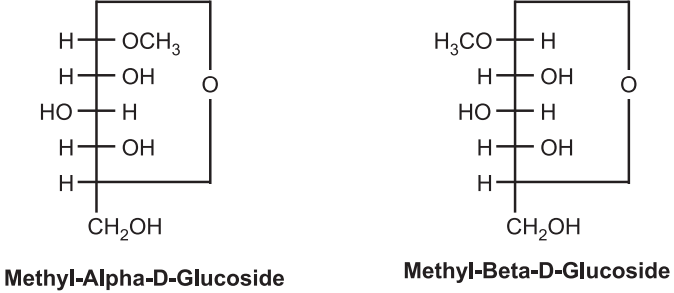
Glycosides: Glycoside is an organic compound that contains C, H, and O in its structure. They are natural carbohydrate substances. They are obtained from maximum higher plants but in less quantity. They are also known as internal acetate. They have two parts viz. Sugar part and non-sugar part. The sugar part is known as the glycone part whereas the non-sugar part is known as the aglycone part or genin part. Due to the presence of the aglycone part, glycosides give therapeutic activities. Sugar and non-sugar parts are linked with glycosidic bridge, known as glycosidic linkage. This linkage is breaks by acid or enzyme hydrolysis and both glycone and genin parts are separated. Glycone part is water-soluble but insoluble in organic solvents whereas aglycone parts are vice-versa. They are formed by the biochemical reaction which makes the water-insoluble compound more polar than the water-soluble molecule. Hence, they are removed from an organic system. Human forms them in the liver as part of the process of detoxification and they are excreted through urine. Mammalian glycosides are simple compounds whereas plant-originated glycosides are much larger and chemically complex. Among the glucose found in nature, D-glucose is available more. They are having two types of stereochemistry namely alpha and beta-glycosides. Example: Methyl D-glucosides.
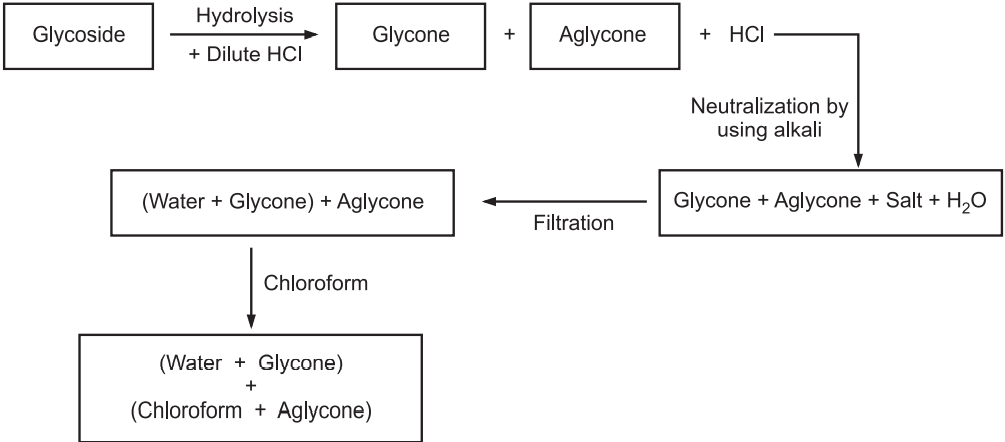
Separation of Glycosides Parts:
Separation is mainly done in the separating funnel. Alcohol and acetone are not used as solvents for aglycone separation because they are water-miscible. The best solvent is ethyl acetate to extract the aglycone part because the solvent is immiscible in water and in a separating funnel the solvent is present in the upper layer (Fig.1).
Physical Properties:
- Glycosides are solid and amorphous powder.
- They are colourless but some are coloured (Except Anthraquinone is red or orange, Flavonoids are yellow).
- They are water-soluble but insoluble in organic solvents.
- They are mostly bitter (Except Glycyrrhizin, Stevioside, Populin).
- They are odourless (Except Saponin glycoside).
- They are non-volatile.
- Many sugar-containing glycosides are insoluble in water but soluble in alcohol.
- They are hydrolysed by mineral acids or enzymes to form glycone and aglycone parts.
- The glycone part is water-soluble whereas the aglycone part is alcohol soluble.
- After hydrolysis, they react with Molash’s reagent and Fehling’s test.
Chemical Properties:
- With acid hydrolysis glycoside is separated into sugar and non-sugar parts. The acetal linkage is more readily cleaved than the linkage between the individual sugars of the sugar chain. But C-glycosides are resistant to acid hydrolysis.
- With strong and mild alkali, they hydrolyse the ester group. They open lactone rings. Example: Cardiac glycosides.
- With enzymatic hydrolysis, sugars are split stepwise from the terminal sugars. Enzymes are specific for some types of glycosides to split. Like Emulsin hydrolyses beta-glycosides, Invertase hydrolyses alpha-glycosides, Myrosin hydrolyses sulphur glycosides etc.
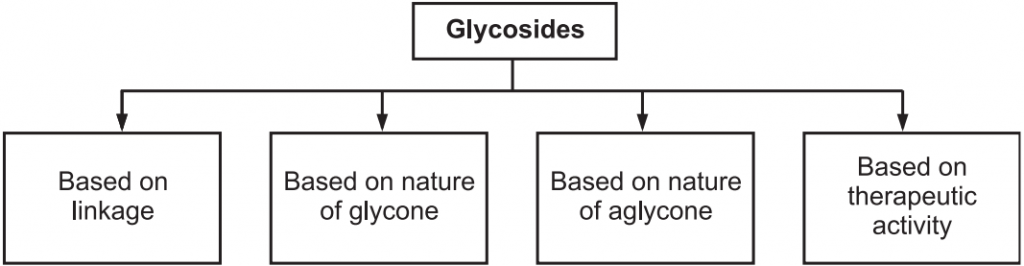
Classification of Glycosides
Table of Contents
Glycosides are the larger group of natural secondary metabolites obtained from many higher plants. They are broadly classified into several groups. They are as follows:
1. Based on Linkage:
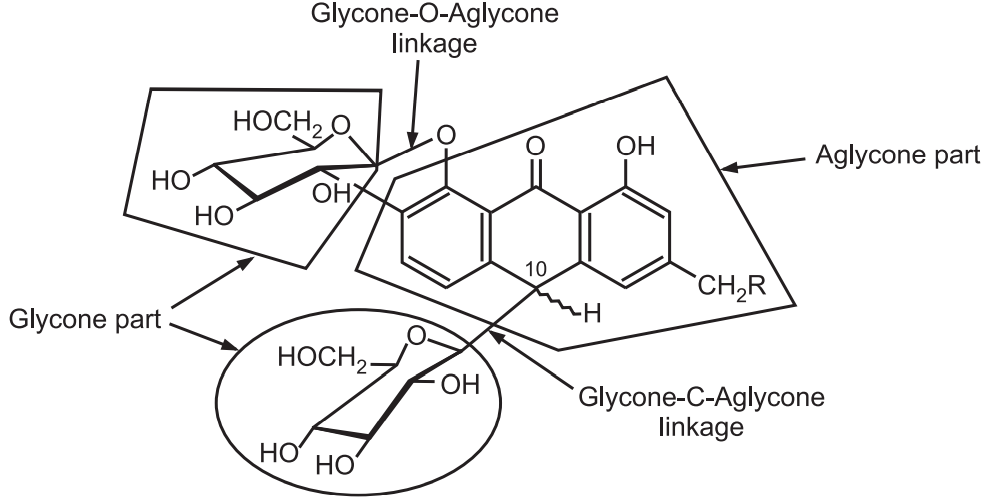
Glycosides are classified based on linkage between glycone and aglycone part where OH groups react with any medicates like, OH, CN, SH, NH product in aglycone part and as per that they are of four types.
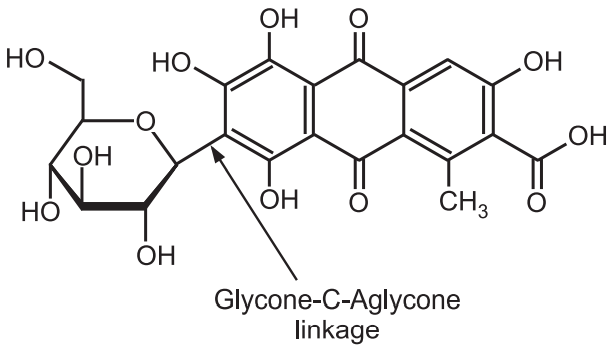
(a) C-glycoside:
Glycone-OH + HC –aglycone → Glycone-C-aglycone + H2O
Some of the anthraquinone glycosides like cascaroside in cascara, aloin in aloes show the particular linkage. C-glycosides are called aloin type glycosides present in aloes. They do not hydrolyse by heating with dilute acid or alkalis but occur by oxidative hydrolysis with FeCl3.
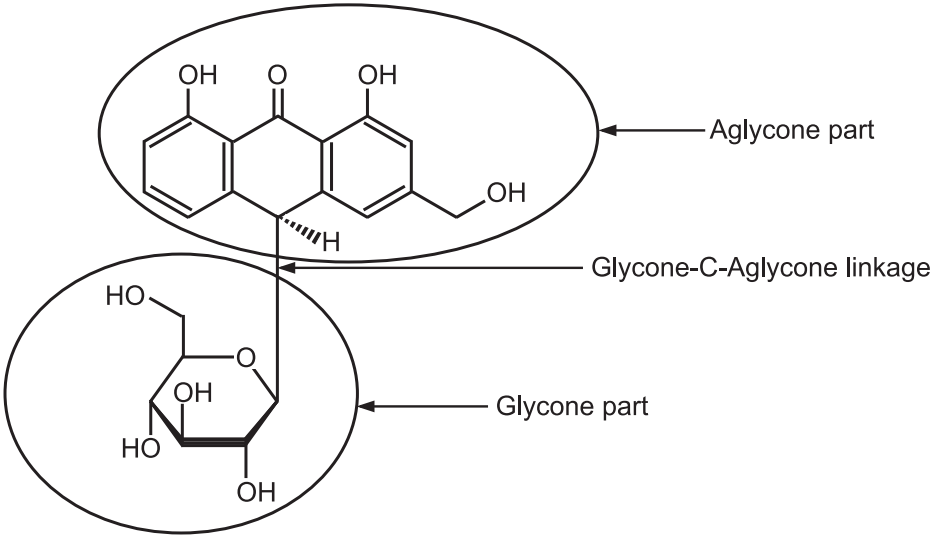
Another example is Carminic acid which is obtained from Cochineal insects (Fig.4).
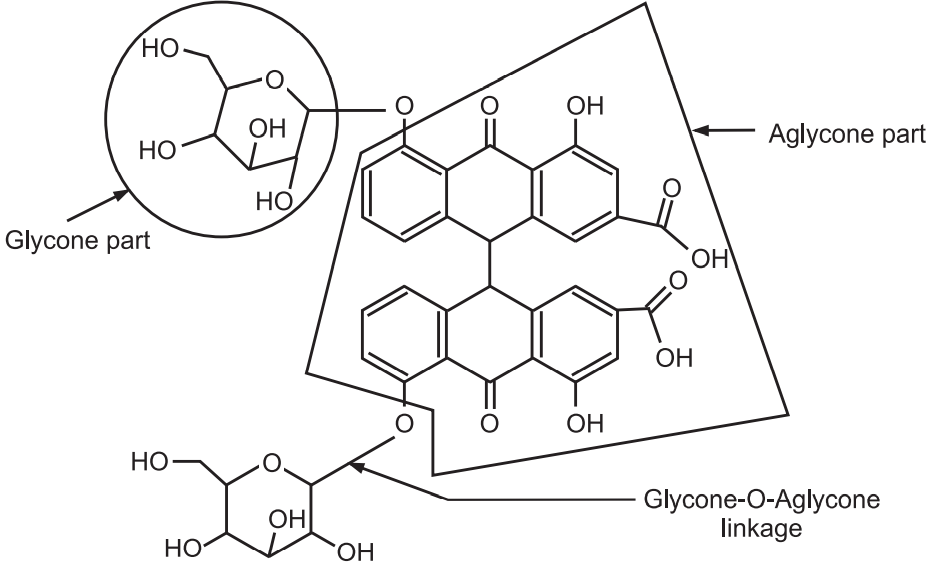
(b) O-glycoside:
Glycone-OH + HO-aglycone → Glycone-O-aglycone + H2O
They are common in higher plants. Example: Senna, Rhubarb (Fig.5). They are hydrolysed by treatment with acid or alkali into glycone and aglycone portions.
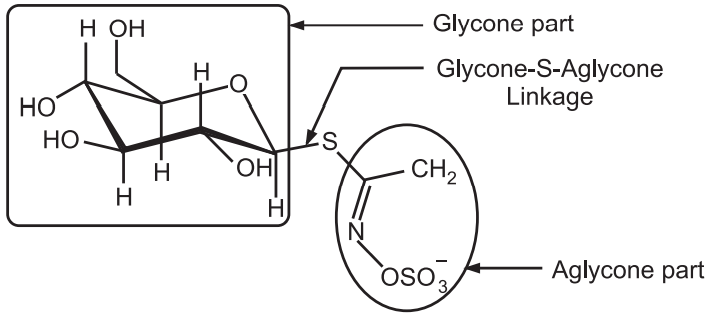
(c) S-glycoside:
Glycone-OH + HS–aglycone → Glycone-S-aglycone + H2O
The occurrence of this glycoside is in isothiocyanate glycoside like sinigrin in black mustard formed by the condensation of sulphhydryl group aglycone to OH group of glycone (Fig.6).
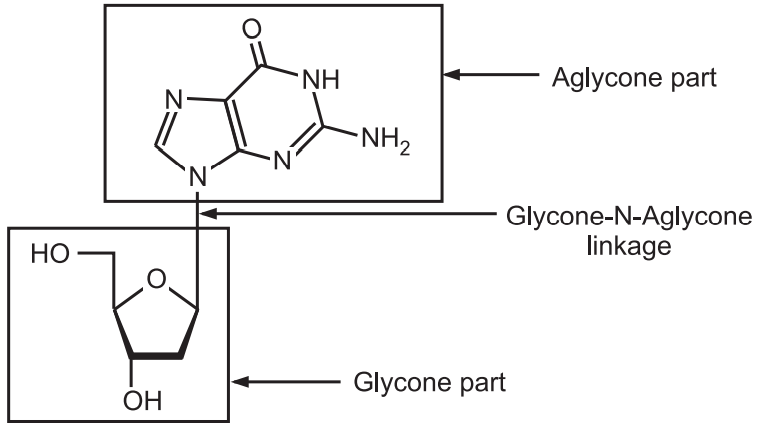
(d) N-glycoside:
Glycone-OH + HN–aglycone → Glycone-N-aglycone + H2O
They are mostly present in the nucleoside where the amino group reacts with the OH group of ribose or deoxyribose resulting in N-glycoside (Fig.7).
(e) C and O-Glycosides:
This is one more type of glycosidic linkage where glucose molecules are attached with the aglycone part by both C and O linkages. Example: Cascarosides from Cascara (Fig.8). Some flavonol glycosides have also contained this type of linkage.
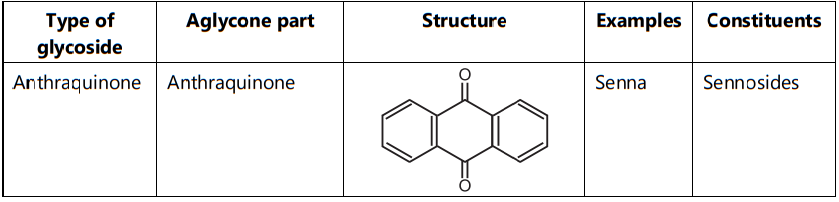
2. Based on the Chemical Nature of Non-sugar Moiety:
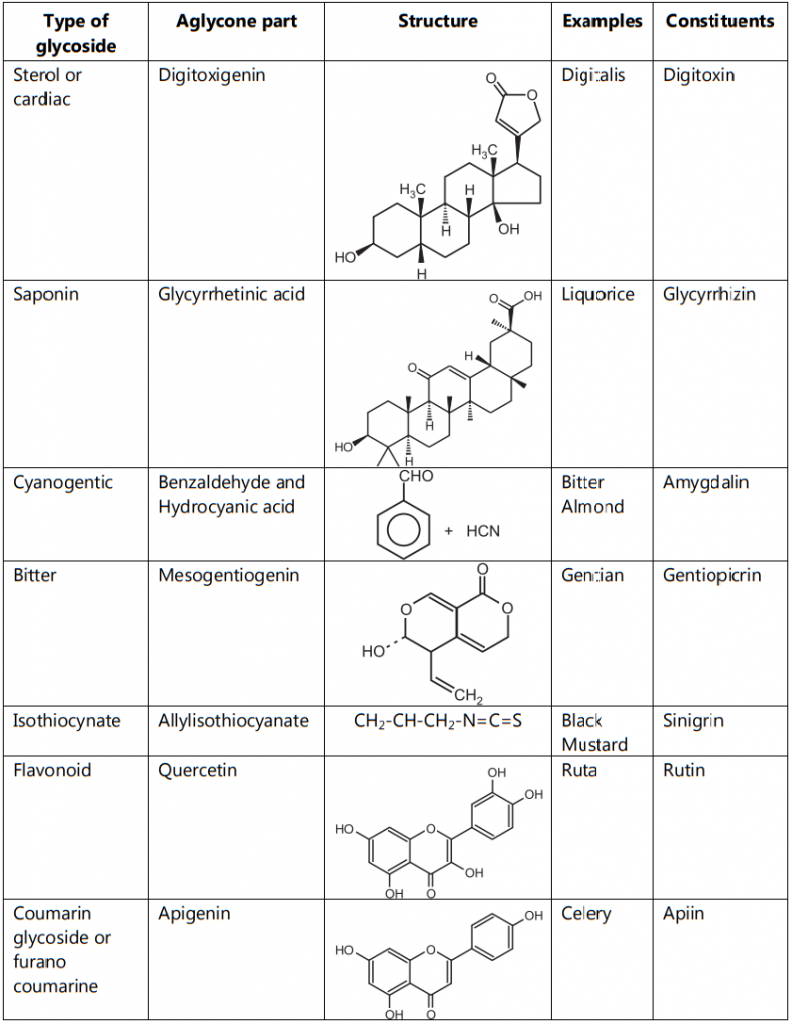
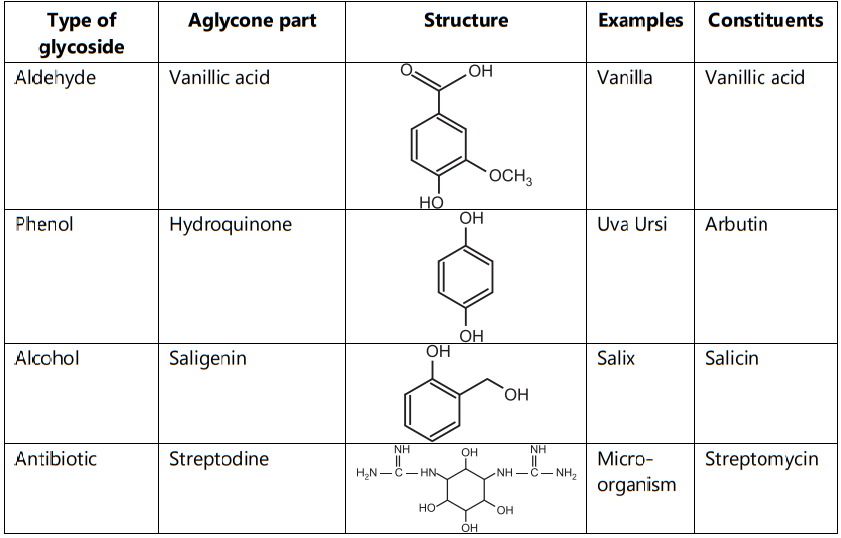
3. Based on the Nature of Sugar Moiety:
- Glucoside: The sugar portion is glucose.
- Rhamnoside: The sugar portion is rhamnose.
- Pentoside sugar portion is pentose.
- Fructoside sugar portion is fructose.
- Arabinoside sugar portion is arabinose.
4. Based on Therapeutic Nature of Glycoside:
- Cardiac glycoside: Examples: Digitalis, Squill.
- Laxative glycoside: Examples: Senna, Aloe.
- Anti-ulcer glycoside: Examples: Liquorice.
- Bitter glycoside: Examples: Chirata, Quassia wood.
- Local irritant: Examples: Black and white mustard.
- Analgesic and antipyretic: Example: Salix bark.
General Extraction:
Stas-otto method: The drug-containing glycoside is finely powdered and subjected to successive extraction in a Soxhlet apparatus with alcohol or suitable solvent. After extraction, the extract is collected and treated with lead acetate to precipitate tannins. Filtered the solution and to the filtrate, H2S gas is passed. The precipitate of lead sulphide form is removed by filtration. The filtrate is subjected to fractional crystallization, distillation or chromatography gives pure glycoside. Further, the molecular structure is determined by the Spectrophotometer, Ultra Red assays, Infra-red, NMR, Mass spectroscopy, etc.
General Chemical Tests:
1. Test for General Glycoside:
Test–A: Treated a few mg of powdered drug with sulphuric acid and then 5% NaOH solution is added for neutralization. Finally, Fehling’s solutions A and B are added to the above mixture. The solution produces a red colour.
Test-B: Dissolved a few mg of powdered drug with sufficient water to make a solution. This solution is tested with Fehling’s solutions A and B. The red colour is produced. This indicates reducing sugar is present in the drug.
Both the red colours in separate test tubes are compared. If the colour of test A is more intense than test B, then glycoside’s presence is confirmed.
2. Test for Anthraquinone Glycoside:
(a) Brontrager’s Test: This test is performed for the O-glycosides. Senna gives a positive test. The powdered drug is dissolved in a few ml dilute sulphuric acid and the mixture is boiled. Filtered the solution, the filtrate is then extracted with an organic solvent like chloroform. The chloroform layer is separated and to that ammonia is added. The ammonia layer gives a rose pink colour. This indicates the presence of O-glycosides.
(b) Modified Brontrager’s Test: This test is performed for the presence of C-glycosides. This test is positive for Aloes. Powdered drug is mixed with dilute hydrochloric acid and FeCl3. This solution converts C-glycoside to O-glycoside. Filtered the solution, the filtrate is then extracted with an organic solvent like chloroform. The chloroform layer is separated and to that ammonia is added. The ammonia layer gives a rose pink colour. This indicates the presence of C-glycosides.
Tests for Cardiac Glycoside:
(a) Keddie’s Test: Chloroform extract of drug mixed with 90% alcohol and 2% 3, 5-dinitrobenzoic acid. Further 7% NaOH is added. The solution turns to blue or violet colour. This confirms the presence of cardenolide aglycone.
(b) Antimony Trichloride Test: To a powdered drug added solution of antimony trichloride and trichloroacetic acid then heated the mixture. The solution appears blue or violet. This indicates the presence of Cardenolides and Bufadienolides.
(c) Keller–Killiani Test: Powdered drug is extracted with chloroform. Then few ml of acetic acid and FeCl3 is added. After that concentrated sulphuric acid is added to the side tube slowly. The acid layer shows a reddish-purple ring. This indicates the presence of deoxy sugar, digitoxose.
(d) Raymond’s Test: Small quantity of powdered drug dissolved in ethanol. In this solution, 1% solution of m-dinitrobenzene, methanol and few drops of sodium hydroxide is added. Violet colour confirms the presence of cardiac glycosides. After standing, the violet colour slowly changes to blue colour. This indicates the presence of the methylene group at the C-21 position in the lactone ring.
(e) Legal’s Test: This test is performed by using pyridine and alkaline sodium nitroprusside. The solution produces a red colour. This indicates the presence of cardiac glycoside.
(f) Baljet Test: Picric acid and sodium picrate are added to the powdered drug sample. The solution becomes orange in colour. This indicated the presence of cardiac glycoside.
3. Test for Cyanogenetic Glycoside:
(a) Sodium Picrate Test: Drug is mixed with dilute sulphuric acid. After the addition of sodium picrate red colour is produced. This indicates the presence of cyanogenetic glycoside.
(b) Mercuric Acetate Test: Drug solution is mixed with mercuric acetate and forms drug acetate and mercury is separated. This confirms the presence of cyanogenetic glycoside.
4. Test for Flavonoids:
(a) Shinoda Test: Powdered extract mixed with a few ml of 95% ethanol, few drops of conc. HCl and 0.5 g magnesium turnings. The pink colour was observed. It indicates the presence of flavonoids.
(b) To the extract few ml of lead acetate solution is added. The yellow coloured precipitate is formed. The addition of an increasing amount of sodium hydroxide to the residue shows yellow colour but becomes colourless after the addition of acid.
5. Test for Triterpenoid:
Few mg of dried extract dissolved in acetic anhydride heated to cool. Then few ml of concentrated sulphuric acid is added along the side tube. The formation of pink colour indicates the presence of triterpenoid.
Salkowski Test: The extract is treated with few drops of concentrated sulphuric acid, red colour at the lower layer indicates the presence of steroids and the formation of a yellow coloured lower layer indicates the presence of triterpenoids.
6. Test for Saponins:
(a) Froth Formation Test (Foam): The solution of drug placed in water in a test tube, shake well, stable froth (foam) is formed.
(b) Haemolysis Test: Solution of saponin (prepared in 1% normal saline) sample is added to few ml of blood in normal saline and mixes well. Centrifuged and note the red supernatant formed which compare with the control tube containing a few ml of 10% blood in normal saline diluted with normal saline.
Functions of Glycoside
In Plants:
- They convert toxic materials into a non-toxic forms.
- They transfer water-insoluble substances by using monosaccharides.
- They are the sources of energy by storage of sugar.
- They store harmful plant products such as phenol.
- They regulate growth.
- Some glycosides have antibacterial activity so they protect the plant from bacterial and other diseases.
In Animals:
- Glycosides have wide groups of chemical natures due to that they are used as many therapeutic activities. Such as laxative, cardioprotective, analgesic, local irritants etc.
- The sugar part in glycoside helps in the solubilisation of non-sugar parts for increasing the bioavailability of the drugs.
- Phenolic glycosides are used as a urinary antiseptic effect.
- Alcohol glycosides are used as analgesic, antipyretic, anti-inflammatory action.
- Cardiac glycosides are used for heart disease.
- Bitter substances have antimicrobial as well as antibiotic actions.
- Thiol glycosides are used as a pain killer.
- Anthraquinone glycosides are used as laxative action.
Make sure you also check our other amazing Article on : Classification of Alkaloids
