Classification of Tannins: Tannins are naturally occurring non-nitrogenous compounds. They belong to water-soluble polyphenols with high molecular weight, ranging from 500 to 3000 (Gallic acid esters) up to 20,000 (Proanthocyanidins). The first time the term tannin is coined by Seguin in 1796. They have several hydroxyls and carboxyl groups to form strong complexes with various macromolecules. The tannins enter into the class of semiochemicals which act as messengers within or between species. Based on that, semiochemicals are divided into two classes viz. pheromones and allelochemicals. Pheromones are involved in the communication between the same species while allelochemicals interact with different species. They are widely distributed in many higher plant species such as Aceraceae, Actinidiaceae, Bixaceae, Burseraceae, Combretaceae, Ericaceae, Myricaceae (Dicot plants) and Najadaceae, Typhaceae (Monocot plants) families. They are mainly located in the vacuoles or surface wax of plants. They occur normally in the roots, wood, bark, leaves, and fruits of many plants. They also occur in galls as pathological growths prevent insect attacks.
Physical Properties:
- Tannins are dark brown or reddish-brown.
- They are amorphous, non-crystalline.
- They are available in the form of powder, flakes, or spongy mass.
- They are water, alkali, alcohol soluble but sparingly soluble in chloroform and other organic solvents.
- They form a colloidal solution with water.
- They form a protective coating in place of wound injury.
- They have an astringent taste.
- They combine with skin and hide to form leather. They react with gelatine to form an insoluble compound.
Chemical Properties:
- Tannins form precipitation with proteins, gelatine, alkaloids, etc.
- They have antioxidant properties due to the presence of polyhydroxy phenolic compounds.
- They have astringent properties due to the formation of precipitation with proteins.
- They yield purple, violet, or black precipitate with iron compounds. Examples: Hydrolysable tannins react with ferric salt to form blue-black precipitation whereas condensed tannins from brownish green precipitation with the same.
- They react with potassium ferricyanide in presence of ammonia to form a deep red color solution.
- They are precipitated by metallic salts like potassium dichromate and lead acetate and sub-acetate.
- They are used in the clarification of wine and beer.
- As a constituent, it reduces the viscosity of drilling mugs for oil wells.
Classification of Tannins
Table of Contents
Based on the identity of phenolic nuclei and the linkage, tannins are classified into two main categories (Fig.1).
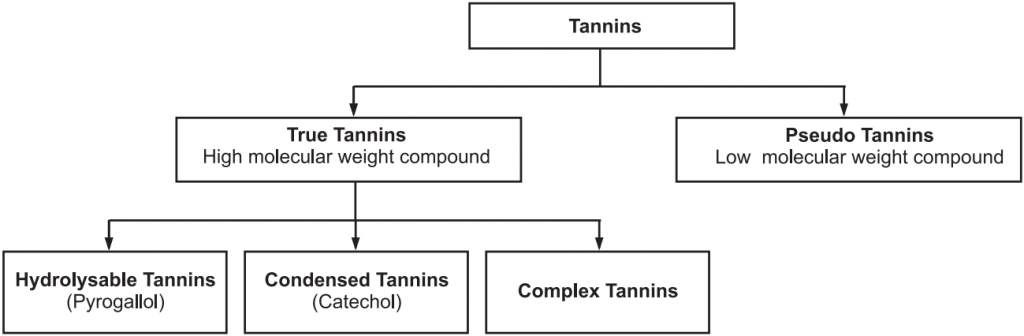
(a) Hydrolysable Tannins:
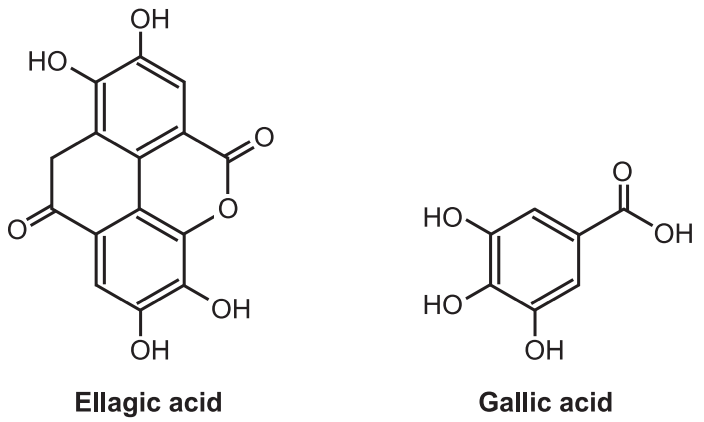
These tannins are esters of sugar mainly glucose with one or more trihydroxy benzene carboxylic acid. These tannins are hydrolyzed by acids, or enzymes (Tannase). Their structures are composed of several polyphenolic acid molecules such as gallic acid and ellagic acid which are bound through an ester linkage to a central glucose molecule. Based on the phenolic acid produced, hydrolyzable tannins are classified into gallotannins and Ellagitannins.
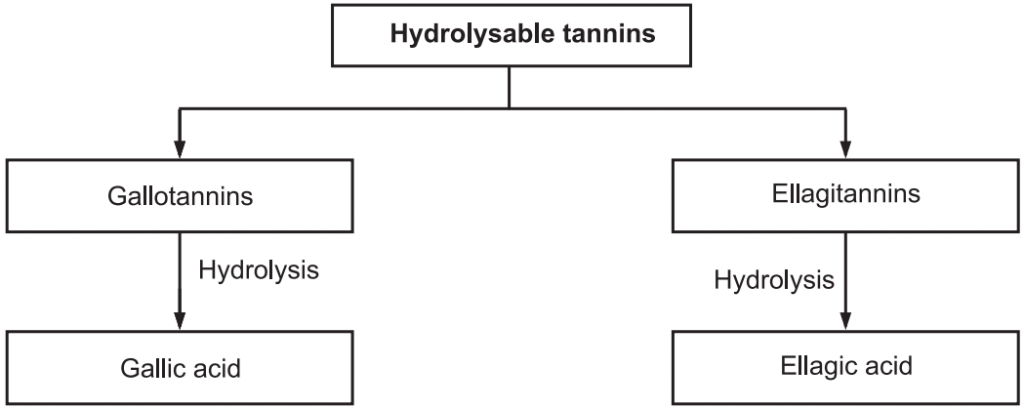
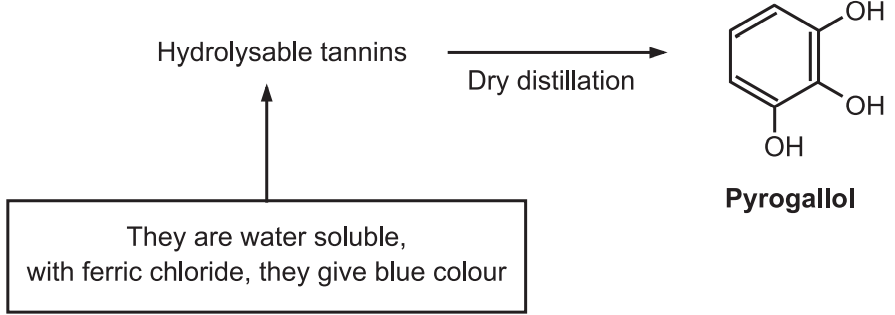
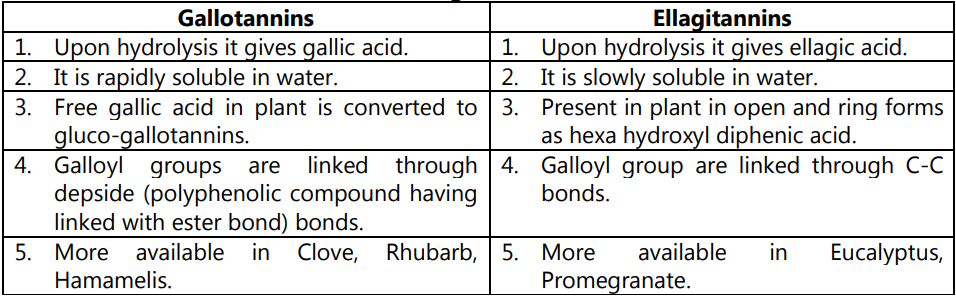
Therefore, gallic acid is a hydrolyzable product of gallic tannins and ellagic acid is a hydrolyzable product of ellagitannins. The gallic acid is found in rhubarb, clove whereas ellagic acid is found in eucalyptus leave and myrobalans, and pomegranate bark. These tannins were treated with ferric chloride to produced blue or black color. Gallitannins are rapidly soluble in water whereas ellagitannins are slowly soluble in water.
Gallotannins are mainly extracted from Tara (Caesalpinia Spinosa), sumac (Rhus coriaria), and gallnuts (Quercus infectoria and Rhus semialata). The ellagitannins, made from ellagic acid glycosides, are one of the components of oak wood (Quercus robur, Quercus petraea, and Quercus alba), chestnut wood (Castanea sativa), and myrobalan (Terminalia schedule).
Hydrolyzable tannins are also known as pyrogallol tannins as the component of phenolic acid on dry distillation converts pyrogallols and phenolic compounds.
Hydrolyzable tannins: Examples: Myrobalan, Bahera, Amla, Arjuna.
(b) Condensed Tannins:
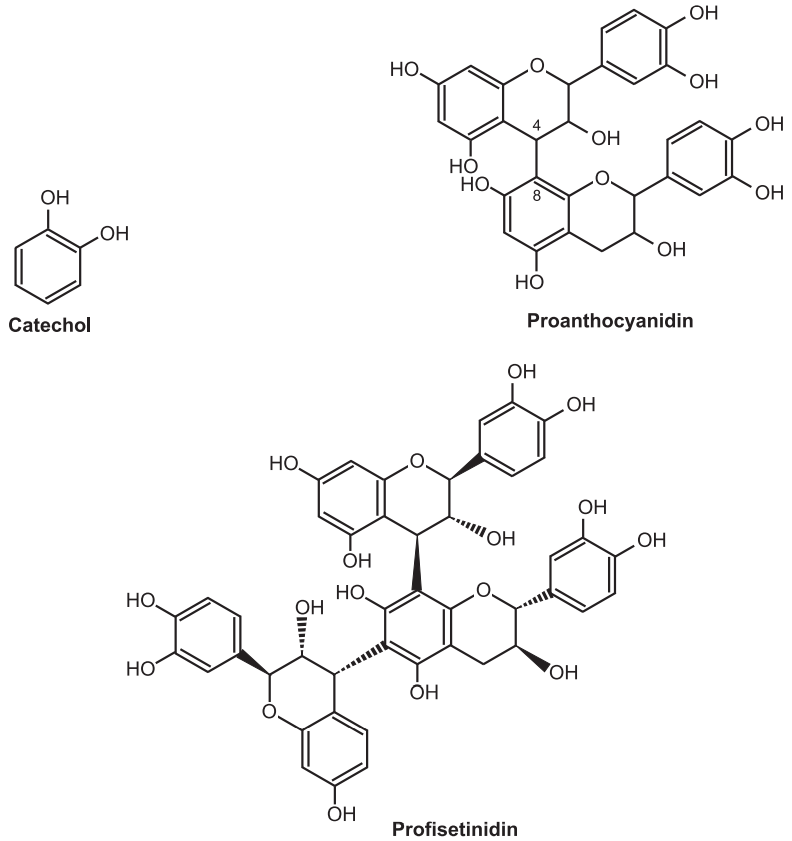
These tannins are resistant to hydrolysis (do not contain sugar moiety) and they are derived from the flavonols, catechins, and flavan-3, 4-diols. Catechin occurs with tannins and flavan-3, 4-diols are intermediates in the biosynthesis of polymeric molecules. On treatment with acids or enzymes they are decomposed into phlobaphenes (Red insoluble compound). On dry distillation they produce catechol hence they are also known as catechol tannins. These tannins are found in cinchona bark, male fern, wild cherry bark, Black catechu, Pale catechu, Ashoka, Pterocarpus, etc. They produce brownish-green color with ferric chlorides. They are further divided into two main categories namely proanthocyanidin and profisetinidin. Proanthocyanidins are oligomeric flavonoids and are naturally present in grapes (Vitis vinifera) consisting of various flavonoids which release anthocyanins and other insoluble molecules when they are treated under acid hydrolysis. They are mainly diffused in the skins and seeds of grapes, and therefore you can find them in red wines. Cocoa beans contain the highest concentrations of Proanthocyanidins. Profisetinidins are formed from leuco-fisetinidin, the leucoanthocyanidin form of fisetinidin. They were extracted from the quebracho wood (Schinopsis lorentzii).
(c) Complex Tannins:
They are the group of tannins that are biosynthesized from both hydrolyzable tannin (C-glucoside ellagitannin) and condensed tannin (Flavono-ellagitannin). Example: Acutissimin. It is prepared by reacting a substance called vescalagin, extracted from oak wood, with a flavanoid from grapes called catechin. Some other examples like tea (Thea Sinensis), Oak (Quercus infectoria), hamamelis (Hamamelis virginiana) leaves and bark, chestnuts (Castanea sativa) contain both hydrolyzable and condense tannins.
(d) Pseudo Tannins:
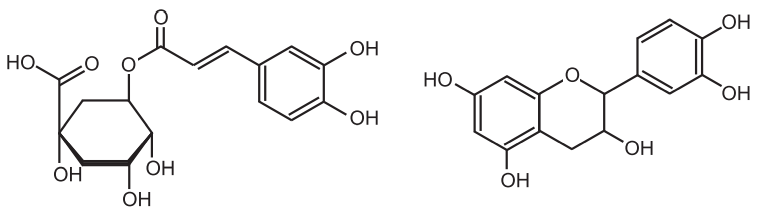
They are a sub-group of tannins because they do not respond to Gold beaters skin test. They have low molecular weight compounds. They are simple phenolic compounds. They are found mainly dead tissues and dying cells of plants. In concentrated solution, they form a precipitate with gelatin. They are found in catechu and nux-vomica, etc. Examples: Chlorogenic acid in coffee and Nux vomica, ipecacuanha acid in Ipecac, catechins in cocoa. Chlorogenic acid is identified by extracting the drug with water followed by oxidation, which produces the green color.
(e) Phloro Tannins:
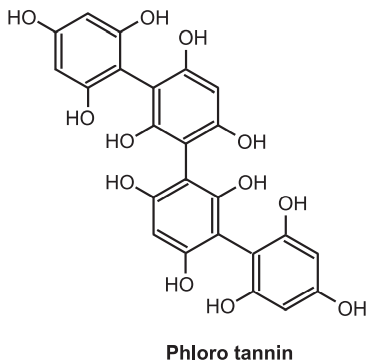
Recently, the third class of tannins has been identified, the phlorotannins, present in many species of dark brown algae such as kelps and rockweeds or sargassacean. It is also available in some red algae. These compounds are oligomers of phloroglucinol (polyphloroglucinols) and based on the arrangement of the phloroglucinol monomers they are further classified. They are known as tannins because they form precipitates by the reaction of proteins. They are distributed in six main sub-groups namely fucols, phlorethols, fucophloretols, fuhalols, and Eckols, which are only found in the Alariaceae family. As per the linkage, they have been classified into four subclasses viz. phlorotannins with an ether linkage (fuhalols and phlorethols, fuhalols are constructed of phloroglucinol units that are connected with para- and ortho-arranged), with a phenyl linkage (fucols), with ether and a phenyl linkage (fucophlorethols) and with a dibenzodioxin linkage in Eckols and carmalols (derivatives of phlorethols containing a dibenzodioxin moiety).
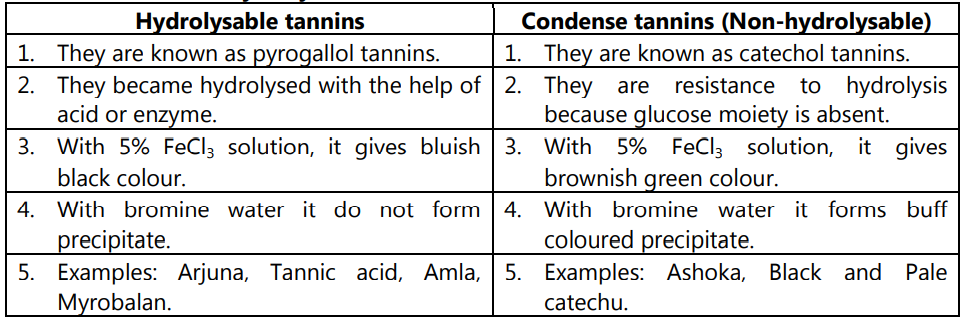
Difference between Hydrolysable and Condense Tannins:
Difference between Gallotannins and Ellagitannins:
Chemical Tests:
1. Gelatin Test: To a solution of tannin, an aqueous solution of gelatin and sodium chloride are added. A white buff-colored precipitate is formed.
2. Goldbeater’s Skin Test: A small piece of goldbeater skin (membrane prepared from the intestine of an ox) is soaked in 20% hydrochloric acid, rinsed with distilled water, and placed in a solution of tannin for 5 minutes. The skin piece is washed with distilled water and kept in a solution of ferrous sulfate. Brown or black color is produced on the skin due presence of tannins.
3. Phenazone Test: A mixture of aqueous extract of a drug and sodium acid phosphate is heated then cooled and filtered. A solution of phenazone is added to the filtrate. A bulky-colored precipitate is formed.
4. Match Stick Test: A match stick is dipped in aqueous plant extract, dried near the burner, and moistened with concentrated hydrochloric acid. On warming near the flame, the matchstick wood turns pink or red due to the formation of phloroglucinol. This test is also known as the Catechin test.
5. Chlorogenic Acid Test: An extract of the chlorogenic acid-containing drug is treated with aqueous ammonia. The green color is formed on exposure to air.
6. Vanillin-hydrochloric Acid Test: Sample solution and added vanillin-hydrochloric acid reagent (Vanillin 1 gm, alcohol 10 ml, concentrated hydrochloric acid 10 ml) a pink or red color is formed due to the formation of phloroglucinol.
General Extraction:
Both hydrolyzable and condensed tannins are water and alcohol-soluble but insoluble in organic solvents. Hence, tannin-containing compounds (Gallic acid, ellagic acid, other tannins) are extracted using water or alcohol as a solvent. Chloroform-containing drugs are removed through ether extraction. Collected aqueous layer and concentrated to get a crude extract of tannins.
Functions:
- Medicinally they are used as antidotes, antiseptics, astringent properties.
- They are used in ink manufacturing industries.
- They are used as preservatives.
- They are used for vegetable tanning.
- They are used to inhibit lipid peroxidation and plasmin.
- They are used for lipolysis in fat cells.
- They are used to treat tonsillitis, pharyngitis, hemorrhoids, and skin eruptions.
Make sure you also check our other amazing Article on : Glycosides
