Complexes or co-ordination compounds result from a donor-acceptor mechanism or Lewis acid-base reaction between two or more different chemical components. The term complexation is used to characterize the covalent or non-covalent interactions between two or more compounds capable of independent existence. The ligand, a molecule, interacts with substrate, the molecule, to form a complex. Drug molecules can form complexes with other small molecules or with macromolecules. Once complexation occurs, the solubility, stability, partitioning, energy absorption and emission, and conductance of the drugs are changed. Drug complexation, therefore, can lead to beneficial properties such as enhanced aqueous solubility and stability. Complexation can also be useful in the optimization of delivery systems and affect the distribution of the drug in the body after systemic administration due to protein binding. Contrary, complexation can lead to poor solubility or decreased absorption of drugs in the body. For certain drugs, complexation with certain hydrophilic compounds can enhance excretion. Overall, complexes can alter the pharmacologic activity of drugs.
Complexes can be divided broadly into two classes depending on whether the acceptor component is a metal ion or an organic molecule; these are classified according to one possible arrangement. Another class, the inclusion/occlusion compounds, involves the entrapment of one compound in the molecular framework of another. Intermolecular forces involved in the formation of complexes are the van der Waals forces of dispersion, dipolar, and induced dipolar types. Hydrogen bonding provides a significant force in some molecular complexes, and co-ordinate covalence is important in metal complexes. Many drugs bind to plasma proteins which have a significant influence on the duration of drug action. Some drugs in the body exist only in a bound form and proper distribution of such drugs into the extravascular part is governed by the process of dissociation of drugs from the complex. The fraction of drugs that can be in free form can vary but may be as low as 1%. The other fraction remains in associated form as a complex with the protein. The free form of the drug is pharmacologically active and is responsible for action on the body. Thus, the protein binding features of the drug play a significant role in its therapeutic actions.
Classification of Complexation
Table of Contents
Based upon the type of interaction, ligand-substrate complexes are classified as follows.
(I) Metal ion or coordination complexes
- (a) Inorganic type
- (b) Chelates
- (c) Olefin type
- (d) Aromatic type
- (i) Pi (π) complexes
- (ii) Sigma (σ) complexes
- (iii) Sandwich compounds
(II) Organic molecular complexes
- (a) Quinhydrone type
- (b) Picric acid type
- (c) Caffeine and other drug complexes
- (d) Polymer type
(III) Inclusion or occlusion compounds
- (a) Channel lattice type
- (b) Layer type
- (c) Clathrates
- (d) Monomolecular type
- (e) Macromolecular type
(I) Metal Ion or Co-ordination Complexes
A satisfactory understanding of metal ion complexation is based upon familiarity with atomic structure and molecular forces, and electronic structure as well as hybridization. The coordination complex or metal complex is a structure made up of a central metal atom or ion (cation) surrounded by several negatively charged ions or neutral molecules possessing lone pairs. The ions surrounding the metal are known as ligands. The number of bonds formed between the metal ion and ligand is called as coordination number.
(a) Inorganic Complexes: Ligands are generally bound to a metal ion by a covalent bond and hence called to be co-ordinated to the ion. The interaction between the metal ion and the ligand is known as a Lewis acid-base reaction wherein the ligand (base) donates a pair of electrons (to the metal ion, an acid) to form the coordinate covalent bond. For example, the ammonia molecules in hexamine cobalt (III) chloride, as the compound [Co(NH3)6]3+ ⋅ Cl3 are called the ligands and are said to be coordinated to the cobalt ion. The coordination number of the cobalt ion, or several ammonia groups coordinated to the metal ions, is six. Other complex ions belonging to the inorganic group include [Ag(NH3)2]+, [Fe(CN)6]4-, and [Cr(H2O)6]3+.
Each ligand donates a pair of electrons to form a coordinate covalent bond between itself and the central ion having an incomplete electron shell. For example,
Co3+ + 6:NH3 = [Co(NH3)6]3+
Hybridization plays an important part in coordination compounds in which sufficient bonding orbitals are not ordinarily available in the metal ion. The understanding of hybridization can be acquainted using the example of the quadric valence of carbon. It will be recalled that the ground-state configuration of carbon is
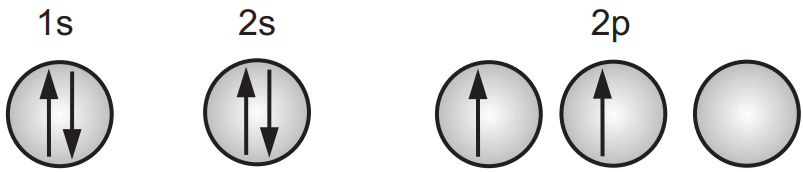
This cannot be the bonding configuration of carbon, however, because it normally has four rather than two valence electrons. Pauling suggested the possibility of hybridization to account for the quadric valence. As per this mixing process, one of the 2s electrons is promoted to the available 2p orbital to yield four equivalent bonding orbitals.
Another example is the interaction between silver and ammonia;
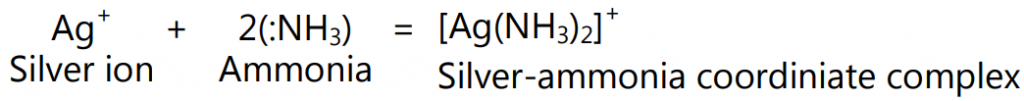
In this case, silver metal ion interacts with ammonia to form a silver-ammonia co-ordinate complex. Electron pair donating ligands such as H2O: NC: Cl: etc neutralize co-ordinate complexes. The [Ag(NH3)2]+ complex is neutralized with Cl as [Ag(NH3)2]Cl. The coordination of compounds through bonds with central metal atoms and surrounding ligands plays important role in controlling the structure and functions of various enzymes in our body.
Co-ordinating a metal to a drug in a non-aqueous system favors the formation of a coordination complex that the resultant coordination complex exhibits a surprising and unexpected buffering effect. Due to buffering effect, the drug can remain soluble in water at physiological pH for a period sufficient for the preparation of a safe and convenient parenteral formulation and for delivering the drug to its targets in the body. Thus, the coordination complexes resolve the problems associated with drugs having poor water solubilities that could not safely be converted to injectable forms or that show declined bioavailability due to their inabilities to migrate to their target sites in the predetermined time. The additional coordination of a buffering ligand or adjuvant to a metal complexed with a drug provides additional buffering capacity and lowers the pH and/or increases the solubility of the entire metal coordination complex.
(b) Chelates: A substance containing two or more donor groups may combine with a metal to form a special type of complex known as a chelate. Some of the bonds in a chelate may be ionic or of the primary covalent type, whereas others are coordinated covalent links. When the ligand provides one group for attachment to the central ion, the chelate is called monodentate. For example, pilocarpine behaves as a monodentate ligand toward Co(II), Ni(II), and Zn(II) to form chelates of pseudo tetrahedral geometry.
Chelation holds stringent steric requirements on both metal and ligands. Ions such as Cu(II) and Ni(II), which form square planar complexes, and Fe(III) and Co(III), which form octahedral complexes and can exist in either of two geometric forms. Because of this isomerism, only cis-co-ordinated ligands (ligands adjacent to a molecule) are readily replaced by a reaction with a chelating agent. Vitamin B12 and the hemoproteins are incapable of reacting with chelating agents because their metal is already coordinated in such a way that only the trans-co-ordination positions of the metal are available for complexation. In contrast, the metal ion in certain enzymes, such as alcohol dehydrogenase, which contains zinc, can undergo chelation, suggesting that the metal is bound in such a way as to leave two cis-positions available for chelation.
Applications of chelation
Chlorophyll and hemoglobin, two extremely important compounds, are naturally occurring chelates involved in the life processes of plants and animals. Albumin is the main carrier of various metal ions and small molecules in the blood serum. The amino-terminal portion of human serum albumin binds to Cu(II) and Ni(II) with higher affinity than that of dog serum albumin. This fact partly explains why humans are less susceptible to copper poisoning than are dogs. The binding of copper to serum albumin is important because this metal is possibly involved in several pathologic conditions. The synthetic chelating agent ethylene diamine tetraacetic acid (EDTA) has been used to tie up or sequester iron and copper ions so that they cannot catalyze the oxidative degradation of ascorbic acid in fruit juices and drug preparations. In the process of sequestration, the chelating agent and metal ion form a water-soluble compound. EDTA is widely used to sequester and remove calcium ions from hard water.
(c) Olefin Type: Olefins belong to a family of organic compounds called hydrocarbons. They consist of different molecular combinations of the two elements, carbon and hydrogen. Another name for an olefin is an alkene. Alkenes contain one or more double bonds between the carbon atoms of the molecule. Olefins from different compounds are based on their structure. Some have short chains with only two, three, or four carbons, such as ethylene. Others form long chains or closed ring structures. Some have a combination of both. Alkenes are insoluble and exist in all three states of matter. Some short-chain alkenes are gases at room temperature and pressure. More complicated structures exist as liquids and solids.
Olefin ligands are common in organotransition metal chemistry. The first organotransition metal complex, Zeise’s salt (K[PtCl3(C2H4]·H2O) was an olefin complex. The bonding of an olefin to a transition metal can activate the ligand to electrophilic or nucleophilic attack depending on the nature and charge of the metal center. For example, if there is a high formal charge on the metal center then the olefin is subject to attack by nucleophiles at the face opposite the metal (giving trans addition). Likewise, electron-rich metal centers in low oxidation states are activated for attack by electrophiles at the C-C bond.
(d) Aromatic Type
(i) Pi (π) complexes: The example of Pi complex is the interaction of local anesthetic bupivacaine and its structural analogs such as 2,6-dimethylaniline, and N-methyl-2, 6-dimethylacetamide, and cocaine, with several electron-deficient aromatic moieties. In solution, the anesthetic, its analogs, and cocaine are electron donors and form π-π charge transfer complexes with strong aromatic acceptors. The concentrations of free bupivacaine, its analogs, and cocaine are reduced from solution via binding to aromatic-functionalized silica.
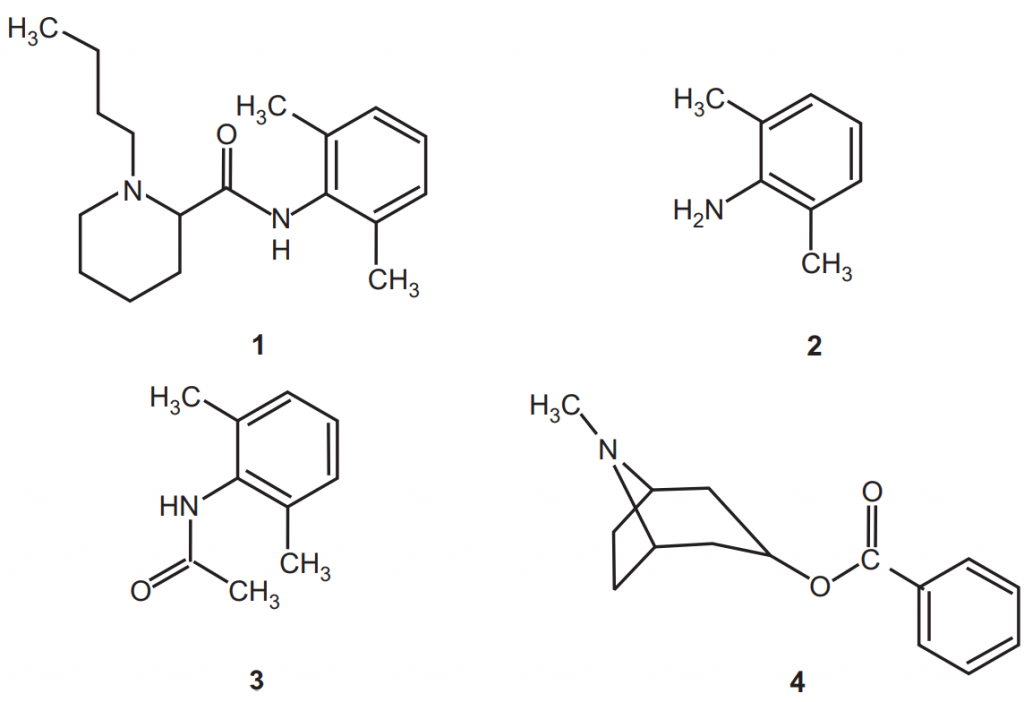
The rapid binding of bupivacaine (1) and its analogs 2, 6-dimethylaniline (2) and 2, 6-dimethyl acetanilide (3), respectively, and of cocaine (4), by the acceptor molecules. Structures 1, 2, 3, and 4 show that the molecules are lipophilic, a characteristic common to toxic molecules. 1, 2, and 3 include a benzene ring with two methyl and nitrogen electron-donating groups, making this portion of the molecules π electron-rich, and hence strong π-donors. The aromatic ring of cocaine, 4, also has weak π-donor capability when complexed with a strong π-acceptor. The selective removal of excess bupivacaine and cocaine from solution is charge transfer complex formation of the π-π type through aromatic-aromatic interaction, based on the assumption that dinitrobenzoyl groups possessing less π-electron density would not only bind with the more π electron-rich bupivacaine and cocaine but would also reduce their toxic effects. The LD50 of bupivacaine is 7.8 mg/kg subcutaneously. The effectiveness of this approach is based on the fact that only free, unbound molecules in the blood possess toxicity and that they lose toxicity once bound to or conjugated with another moiety.
(ii) Sigma (σ) complexes: An arenium ion is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution. This complex is also called a Wheland intermediate or a sigma complex or σ-complex. The smallest arenium ion is the benzenium ion (C6H7+), which is protonated benzene.
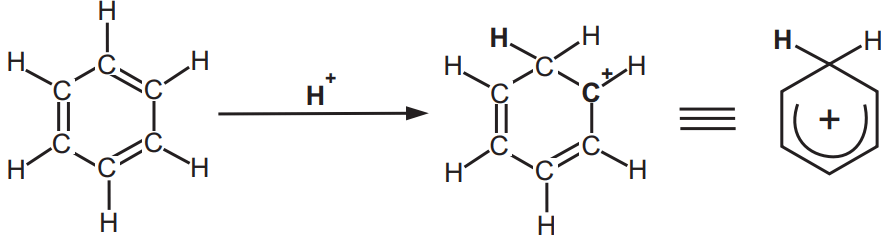
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however, it is relatively stable due to delocalization. The positive charge is delocalized over 3 carbon atoms via the Pi system, as depicted in resonance structures, Fig. 4.
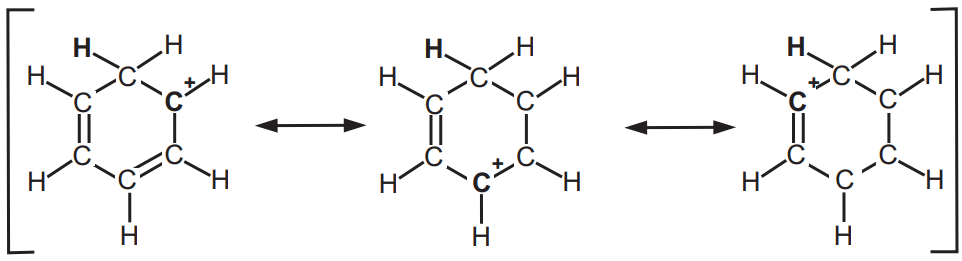
A complexed electrophile can contribute to the stability of arenium ions. A benzenium ion can be isolated as a stable compound when it is protonated by the carborane superacid H(CB11H(CH3)5Br6). The benzethonium salt is crystalline with thermal stability of up to 150 °C. Bond lengths deduced from X-ray crystallography are consistent with a cyclohexadienyl cation structure.
Methylene arenium ion stabilization by metal complexation is another example of σ-complex. In the reaction sequence, the R-Pd(II)-Br starting complex is stabilized by tetramethyl ethylene diamine (TMEDA) which is converted by 1,2-Bis(diphenylphosphino) ethane (DPPE) to metal complex. Electrophilic attack of methyl triflate forms methylene arenium ion with positive charge located in aromatic para position and with the methylene group at 6° out of the plane of the ring. Reaction first with water and then with triethylamine hydrolyzes the ether group.
(iii) Sandwich compounds: A sandwich compound is a metal-bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives (for example Cn(CH3)n), and heterocyclic derivatives (for example BCnHn+1). Because the metal is usually situated between the two rings, it is said to be “sandwiched”. Special classes of sandwich complexes are the metallocenes. Metallocenes including just one facially bound planar organic ligand instead of two give rise to a still larger family of half-sandwich compounds. The most famous example is probably methylcyclopentadienyl manganese tricarbonyl. Compounds such as the cyclopentadienyl iron dicarbonyl dimmer and cyclopentadienyl molybdenum tricarbonyl dimer can be considered a special case of half-sandwiches, except that they are bimetallic.
(II) Organic Molecular Complexes
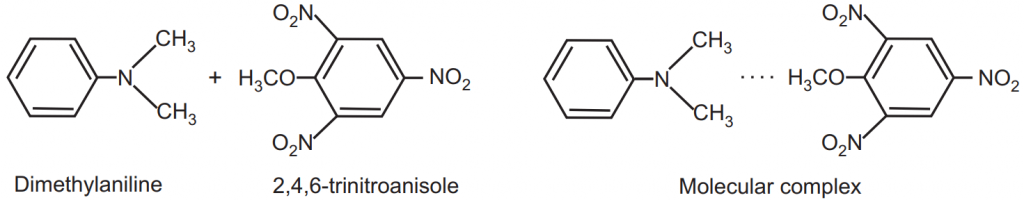
An organic molecular complex consists of constituents held together. The forces involved are of donor and acceptor type or by hydrogen bonds. There is a difference between complexation and the formation of organic compounds. For example, dimethylaniline and 2,4,6-trinitroanisole react at low temperatures to give a molecular complex. The dotted line in the complex, Fig.5, indicates that the two molecules are held together by a weak secondary valence force. It is not to be considered as a clearly defined bond but rather as an overall attraction between the two aromatic molecules. The type of bonding existing in molecular complexes in which hydrogen bonding plays no part is not fully understood, but it may be considered for the present as involving an electron donor-acceptor mechanism corresponding to that in metal complexes but ordinarily much weaker.
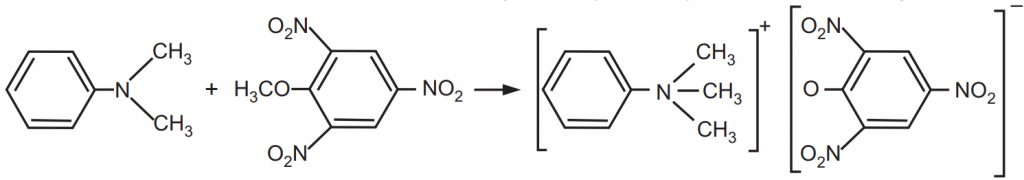
These two compounds react at a higher temperature to form a salt wherein the constituent molecules in products are held together by primary valence bonds, Fig.6.
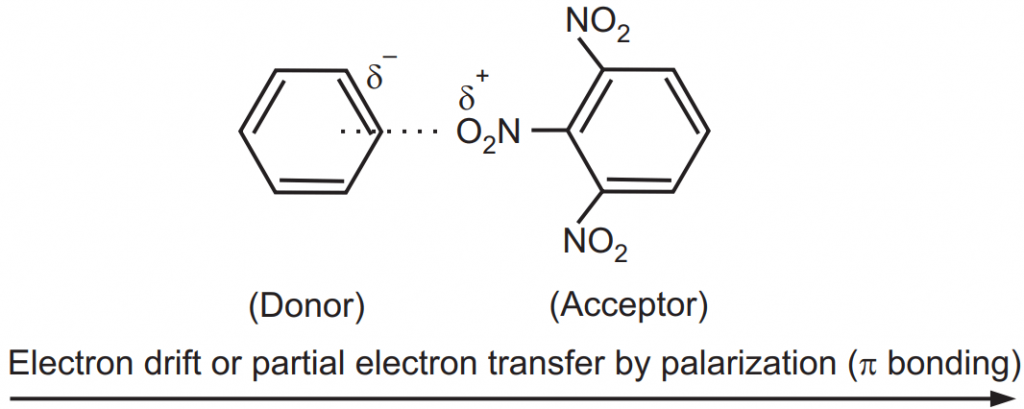
Some of the organic complexes are too weak and cannot be separated as definite compounds. They are even difficult to detect by any chemical and physical means. The energy of attraction between the constituents is approximately ˂5 kcal/moles and the bond
distance is usually greater than 3A°. One molecule of complex polarizes the other to form ionic interaction or charge transfer. Such molecular complexes are referred to as charge-transfer complexes. For example, the polar nitro groups of trinitrobenzene induce a dipole in the readily polarizable benzene molecule. The net electrostatic interaction results in complex formation as shown in Fig.7.
The drug used against alcohol addiction (disulfiram), a sedative-hypnotic and anticonvulsant (clomethiazole), and an antifungal agent (tolnaftate), each of these drugs possess a nitrogen-carbon-sulfur moiety. A complex may form from the transfer of charge from the pair of free electrons on the nitrogen and/or sulfur atoms of these drugs to the antibonding orbital of the iodine atom. The tying up iodine by the molecules containing the N−C=S moiety inhibits thyroid action in the body.
Drug Complexes
In the formation of a drug complex degree of interaction depends upon certain effects. For example, the complexing of caffeine with several acidic drugs. The interaction between caffeine and sulfonamide or barbiturate is a dipole-dipole force or hydrogen bonding between the polarized carbonyl groups of caffeine and the hydrogen atom of the acid. The secondary interaction occurs between the non-polar parts of the molecules and the resultant complex is “squeezed out” of the aqueous phase due to the great internal pressure of water.
The complexes formed between esters and amines, phenols, ethers, and ketones have been attributed to the hydrogen bonding between the nucleophilic carbonyl oxygen and active hydrogen. There are no activated hydrogens on caffeine; the hydrogen at the number 8 position is very weak (Ka = 1 × 10-14) and is not likely to enter complexation, Fig.8. The complexation occurs due to the dipole-dipole interaction between the nucleophilic carboxyl oxygen of benzocaine and the electrophilic nitrogen of caffeine.
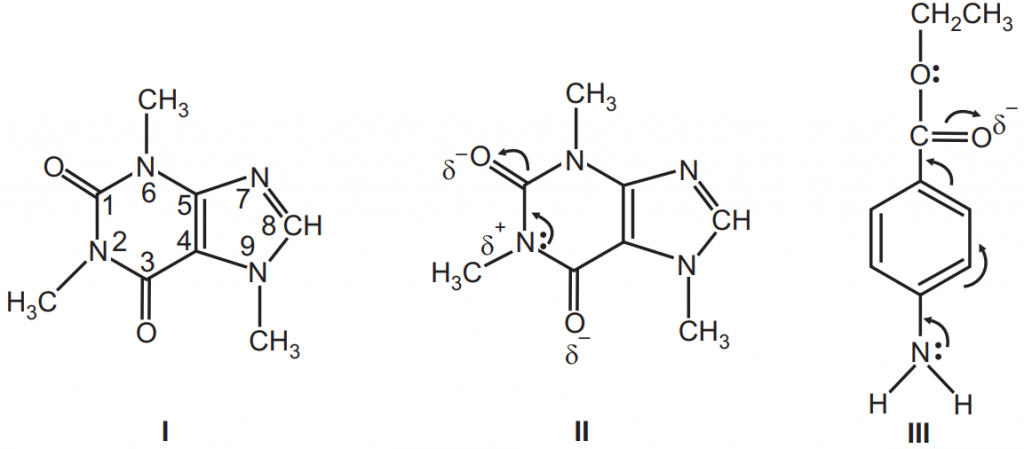
Caffeine forms complexes with organic acid anions that are more soluble than the pure xanthine, but the complexes formed with organic acids, such as gentisic acid, are less soluble than caffeine alone. Such insoluble complexes provide caffeine in a form that masks its normal bitter taste and serves as a suitable state for chewable tablets. Salicylates form molecular complexes with benzocaine. Complexation between benzocaine and salicylates improves or impair drug absorption and bioavailability. The presence of sodium salicylate significantly influences the release of benzocaine, depending on the type of vehicle involved.
Polymer Complexes
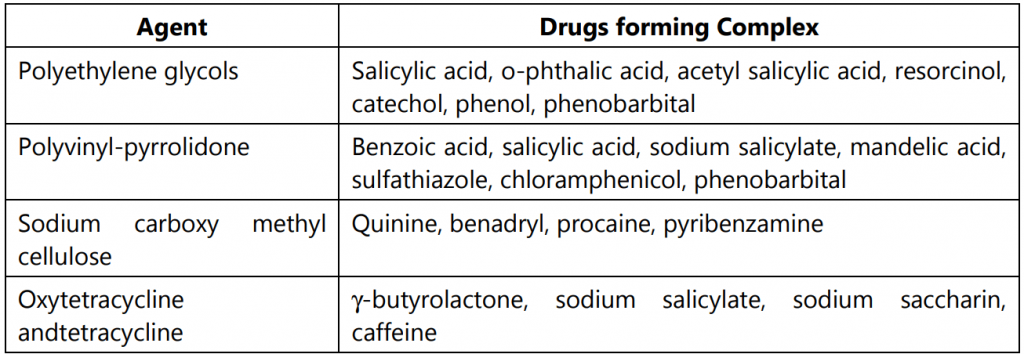
The polymers containing nucleophilic oxygens such as polyethylene glycols, polystyrene, carboxymethylcellulose and similar can form complexes with various drugs. Examples of this type include incompatibilities of carbowaxes, pluronic, and tweens with tannic acid, salicylic acid, and phenol. The interactions may occur in suspensions, emulsions, ointments, and suppositories and are manifested as a precipitate, flocculate, delayed biologic absorption, loss of preservative action, or other undesirable physical, chemical, and pharmacological effects. The interaction of povidone (PVP) with ionic and neutral aromatic compounds is affected by several factors that affect the binding to PVP of substituted benzoic acid and nicotine derivatives. Ionic strength has no influence but the binding increases in phosphate buffer solutions and decreases as the temperature is raised. Crospovidone, a cross-linked insoluble PVP, can bind drugs owing to its dipolar character and porous structure. There exists an interaction of crospovidone with acetaminophen, benzocaine, benzoic acid, caffeine, tannic acid, and papaverine hydrochloride. This interaction is mainly due to any phenolic groups on the drug. Hexyl resorcinol shows exceptionally strong binding.
Solutes in parenteral formulations may migrate from the solution and interact with the wall of a polymeric container. The ability of a polyolefin container to interact with drugs depends linearly on the octanol-water partition coefficient of the drug. For parabens and drugs that exhibit significant hydrogen bond donor properties, a correction term related to hydrogen-bond formation is needed. Polymer-drug container interactions may result in loss of the active component in liquid dosage forms. Such complexes are used to modify biopharmaceutical parameters of drugs; the dissolution rate of ajmaline is enhanced by complexation with PVP. The interaction is due to the aromatic ring of ajmaline and the amide groups of PVP to yield a dipole–dipole-induced complex. Some molecular organic complexes of interest to the pharmacist are given in Table.1.
Inclusion Compounds
The inclusion or occlusion compounds result from the architecture of molecules. One of the constituents of the complex is trapped in the open lattice or cage-like crystal structure of the other to yield a stable arrangement.
Channel Lattice Type
The bile acids especially cholic acids form a complex of deoxycholic acid in combination with paraffin, organic acids, esters, ketones, and aromatic compounds and with solvents such as ether, alcohol, and dioxane. The crystals of deoxycholic acid are arranged to form a channel into which the complexing molecule can fit. Camphor has been partially resolved by complexation with deoxycholic acid, and dl-terpineol using digitonin, which occludes certain molecules in a manner like that of deoxycholic acid. Urea and thiourea also crystallize in a channel-like structure permitting enclosure of unbranched paraffin, alcohols, ketones, organic acids, and other compounds. The well-known starch–iodine solution is another example of a channel-type complex consisting of iodine molecules entrapped within spirals of the glucose residues. Monostearin, an interfering substance in the assay of dienestrol, could be extracted easily from dermatologic creams by channel-type inclusion in urea. Urea inclusion might become a general approach for the separation of long-chain compounds in assay methods.
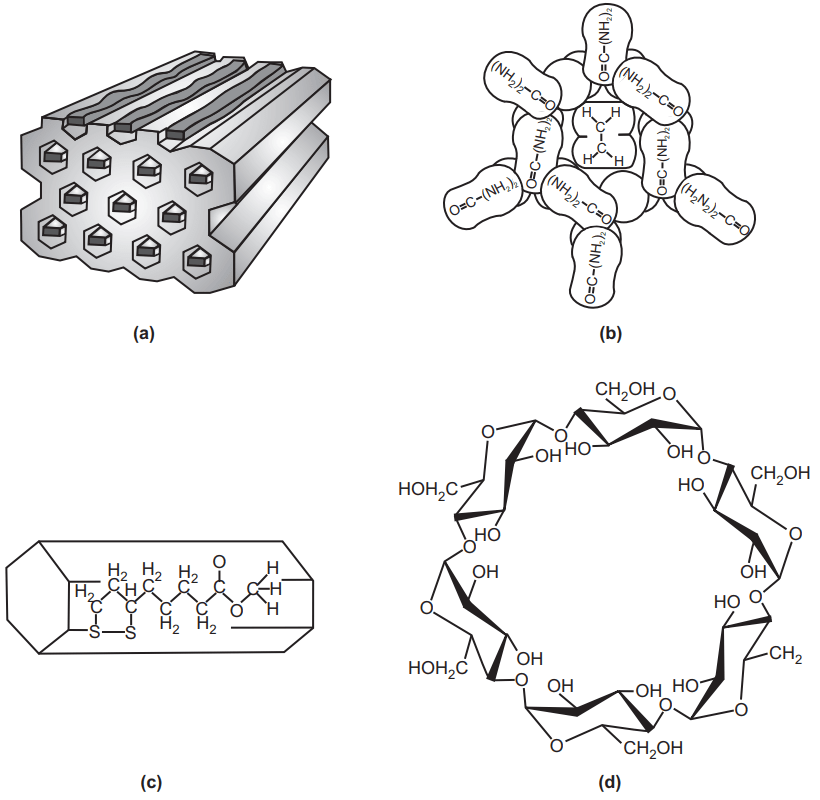
In Fig.9 a channel complex formed with urea molecules as the host. (a) These molecules are packed in an orderly manner and held together by hydrogen bonds between nitrogen and oxygen atoms. (b) The hexagonal channels, approximately 5 A° in diameter, provide room for guest molecules such as long-chain hydrocarbons. A hexagonal channel complex (adduct) of methyl α-lipoate and 15 g urea in methanol (c) is prepared with gentle heating. Needle crystals of adduct separated overnight at room temperature. This inclusion compound or adduct begins to decompose at 63 °C and melts at 163 °C. Thiourea may also be used to form the channel complex. Cyclodextrin (d) is another example of this type.
Layer Type
Some other examples include clay montmorillonite, the principal constituent of bentonite, which can trap hydrocarbons, alcohols, and glycols between the layers of their lattices. Graphite can also intercalate compounds between its layers.
Clathrates
The clathrates crystallize in the form of a cage-like lattice in which the coordinating compound is entrapped. Chemical bonds are not involved in these complexes, and only the molecular size of the encaged component is of importance. The stability of a clathrate is due to the strength of the structure. The highly toxic agent hydroquinone (quinol) crystallizes in a cage-like hydrogen-bonded structure. The holes have a diameter of 4.2°A and permit the entrapment of one small molecule to about every two quinol molecules. Small molecules such as methyl alcohol, CO2, and HCl may be trapped in these cages, but smaller molecules such as H2 and larger molecules such as ethanol cannot be accommodated. Clathrates may be used to resolve optical isomers and to bring about other processes of molecular separation. The warfarin sodium USP, is a clathrate of water, isopropyl alcohol, and sodium warfarin in the form of a white crystalline powder.
(III) Monomolecular Inclusion Compounds:
Cyclodextrins Inclusion compounds are of the channel – and cage-type (clathrate) and mono- and macromolecular type. Monomolecular inclusion compounds involve the entrapment of a single guest molecule in the cavity of one host molecule. Monomolecular host structures are represented by cyclodextrins (CD). These compounds are cyclic oligosaccharides containing a minimum of six dextro-glucopyranose units attached by α-1,4 linkages produced by the action on the starch of Bacillus macerations amylase. The natural α−, β−, and γ− cyclodextrins consist of six, seven, and eight units of glucose, respectively.
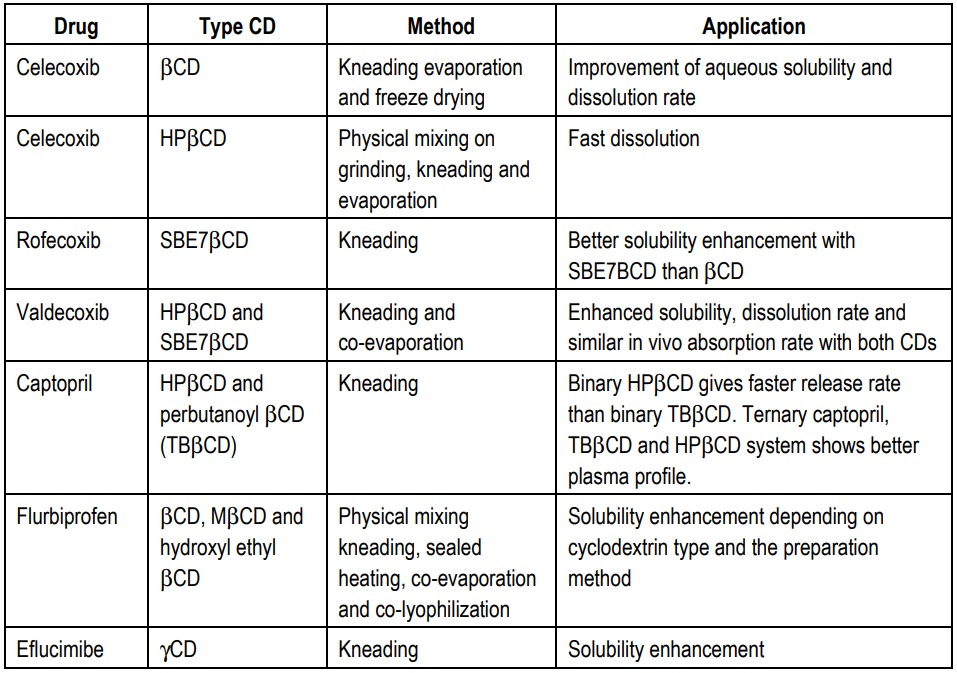
Cyclodextrins are cyclic oligomers of glucose that can form water-soluble inclusion complexes with small molecules and portions of large compounds. These complexes are biocompatible and do not elicit any immune responses and have low toxicities in animals and humans. Some examples of cyclodextrins used in therapeutics along with their method of preparation are listed in Table.2.
Cyclodextrins have wide pharmaceutical applications such as improvement in the bioavailability of drugs of specific interest and delivery of nucleic acids. The CD can form inclusion compounds in an aqueous solution due to the typical arrangement of the glucose units. The cyclodextrin structure forms a doughnut ring. The molecule exists as a truncated cone that can accommodate molecules such as mitomycin C to form inclusion compounds. The interior of the cavity is relatively hydrophobic because of the CH2 groups, whereas the cavity entrances are hydrophilic due to the presence of the primary and secondary hydroxyl groups. The α-CD has the smallest cavity (id 5°A), β-CD and γ-CD has larger cavity size (id 6°A and 8°A, respectively) and are the most useful for pharmaceuticals. The water inside the cavity tends to be squeezed out and to be replaced by more hydrophobic species. Thus, molecules of appropriate size and stereochemistry can be included in the cyclodextrin cavity by hydrophobic interactions. Complexation does not ordinarily involve the formation of covalent bonds. Some drugs may be too large to be accommodated totally in the cavity. Mitomycin C interacts with γ-CD at one side of the torus. Thus, the aziridine ring of mitomycin C is protected from degradation in acidic solution. The inclusion of indomethacin with β-CD is detected using a 1H-NMR technique. The p-chloro benzoyl part of indomethacin enters the β-CD ring, whereas the substituted indole moiety is too large for inclusion and rests against the entrance of the CD cavity.
The complex formation has been used to alter the physicochemical and biopharmaceutical properties of the drug. The complex drug may have altered stability, solubility, molecular size, partition coefficient, and diffusion coefficient. It is used in the various types of poisonings as well as in enhancing drug absorption and bioavailability from the various dosage form. Cyclodextrins are used to trap, stabilize, and solubilize sulfonamides, tetracyclines, morphine, aspirin, benzocaine, ephedrine, reserpine, and testosterone.
Application of Complexation
(i) Solubility enhancement: The aqueous solubility of retinoic acid (0.5 mg/L), a drug used topically in the treatment of acne, is increased to 160 mg/L by complexation with β-CD. Derivatives of the natural crystalline CD have been developed to improve aqueous solubility and to avoid toxicity. Partial methylation (alkylation) of some of the OH groups in CD reduces the intermolecular hydrogen bonding, leaving some OH groups free to interact with water, thus increasing the aqueous solubility of CD. A low degree of alkyl substitution is preferable. Derivatives with a high degree of substitution lower the surface tension of water, and this has been correlated with the hemolytic activity observed in some CD derivatives. Amorphous derivatives of β-CD and γ-CD are more effective as solubilizing agents for sex hormones than the parent cyclodextrins. The relatively low aqueous solubility of the CD is due to the formation of intramolecular hydrogen bonds between the hydroxyl groups, which prevent their interaction with water molecules.
(ii) Bioavailability enhancement: Dissolution rate plays an important role in the bioavailability of drugs, fast dissolution usually favors absorption. The dissolution rates of famotidine (used in the treatment of gastric and duodenal ulcers) and that of tolbutamide (oral antidiabetic drug) are increased by complexation with β-CD. The testosterone complex with amorphous hydroxypropyl β-CD allows efficient transport of hormone into the circulation upon sublingual administration. This route avoids metabolism in the intestines and first-pass decomposition in the liver and thus improves bioavailability.
(iii) Modifying reactivity: Cyclodextrins may increase or decrease the reactivity of the guest molecule depending on the nature of the reaction and the orientation of the molecule within the CD cavity. For example, α-cyclodextrin favors pH-dependent hydrolysis of indomethacin in an aqueous solution, whereas β-CD inhibits it. The water solubility of β-CD (1.8 g/100 mL at 25°C) is insufficient to stabilize drugs at therapeutic doses. It is associated with nephrotoxicity when CD is administered by parenteral routes.
(iv) Modifying drug release: The hydrophobic forms of β-CD have been found useful as sustained-release drug carriers. The release rate of diltiazem (water-soluble calcium antagonist) was significantly decreased by complexation with ethylated β-CD. The release rate was controlled by mixing hydrophobic and hydrophilic derivatives of CD at several ratios. Ethylated β-CD has also been used to retard the delivery of isosorbide dinitrate, a vasodilator.
(v) Taste masking: Cyclodextrins may improve the organoleptic characteristics of oral liquid formulations. The bitter taste of suspensions of femoxetine (antidepressant) is greatly suppressed by the complexation of the drug with β-CD.
(vi) Administration of therapeutic agents: Some therapeutic agents are administered only as complexes due to physicochemical limitations. For example, iron complex with ferrous sulfate and carbonate and insulin complex with Zn and Vitamin-B12. These complexes reduce the GIT irritation, increase the absorption after oral administration, and cause less irritation at the site of injection.
(vii) Use of ion exchange: Cholestyramine resin (quaternary ammonium anion exchange resin) is used to relieve pruritus, the resin exchanges chloride ion from bile result in increased elimination of bile through feces.
(viii) In diagnosis: Technetium 90 (a radionuclide) is prepared in the form of citrate complex and this complex is used in the diagnosis of kidney function and glomerular filtration rate. Squibb (complex of a dye Azure A with carbacrylic cation exchange resin) is used for detection of achlorhydria due to carcinoma and pernicious anemia.
(ix) Complexation as a therapeutic tool: Complexing agents are used for a variety of uses. Many of them are related to the chelation of metal ions. One of the important uses is the preservation of blood. EDTA and citrates are used in in-vitro to prevent clotting. For example, anticoagulant acid citrate dextrose solution and anticoagulant sodium citrate solution. Citrates act by chelating calcium ions in blood as it depletes body calcium.
(x) Treatment of poisoning: Therapeutic procedure involves complexation to minimize poisoning. It is possible by two pathways. First by absorption of toxicants from GIT using complexing and adsorbing agent and second by inactivation of toxic material systemically and enhanced elimination of toxic substance through the use of dialysis. In case of heavy metal poisoning, the basic step involved in detoxification wherein inactivation of metal present in the body is carried out through chelation (metal chelates) and the water-soluble constituents are readily eliminated from the body via the kidney.
(a) Arsenic and mercury poisoning: The most effective agent is BAL (Dimercaprol). The arsenical dimercaprol is shown as CH2SCHSAs-RCH2OH. Two sulfhydryl groups chelate with metal and a free OH group promotes water solubility. BAL is effective in the treatment of poisoning from gold, bismuth, cadmium, and polonium.
(b) Lead poisoning: The treatment of choice for acute/chronic lead poisoning is i.v. administration of calcium or disodium complex of EDTA. This complex chelates ion exhibit a higher affinity of EDTA than do calcium. The route of administration of complex is important and is given only by slow i.v. drip in isotonic NaCl or Sterile 5% dextrose solution. Oral administration promotes the absorption of lead from GIT and increases body levels of lead.
(c) Radioactive materials: Poisoning with radioactive materials particularly with long biological half-life encounters problems that metal has toxic effect and body suffer from radiation damage. Uranium and plutonium exposure have been successfully treated with CaNaEDTA. Plutonium gets deposited and chelate in the bone so, prompt treatment is necessary.
(d) Dialysis and complexation in poisoning: Removal of poisons from systemic circulation can be done by artificial kidney or by peritoneal dialysis. Dialyzing fluid is injected into the peritoneal cavity continually and circulated into and out of the cavity. The toxic material diffuses through the wall of the blood vessel into the fluid present in the cavity. The efficiency of this procedure is improved by using the principle of complexation. If the toxicant is complexed with some high molecular weight non-diffusible component, the rate of dialysis of the toxicant is increased and the complexed toxicant is prevented from returning into the circulation. It is useful in humans and animals. In the treatment of intoxication due to salicylates and barbiturates, serum albumin is commonly used.
Make sure you also check our other amazing Article on : Solubilization
