Cosmetic Excipients: Cosmetics are probably as old as mankind – just like their components. Cosmetic ingredients can generally be derived from a variety of sources, ranging from natural to synthetic substances.
These ingredients are used in the formulation of personal care products which are broadly classified into hair care, skin care, sun care, cleansing, make-up or, oral care, toiletries, cosmetics, and fragrances.
Benefits of Cosmetic Ingredients
Cosmetic ingredients bring many benefits to consumers. For example, UV filters help to protect skin and hair from the harmful effects of UV rays. Surfactants are essential for cleansing and foam building in shampoos and body washes. Preservatives prevent contamination and ensure the longevity of cosmetics and personal care products. A comprehensive list of cosmetic ingredients and their benefits is available on the website of the European Commission.
Regulation of Cosmetic Ingredients
Cosmetic ingredients are globally regulated by regional laws and regulations. In the European Union, they are regulated under the Cosmetics Regulation. The Cosmetics Directive was first adopted in 1976 and has been regularly updated, with its latest update, the entry into force on July 11, 2013, of the Cosmetics Regulation. CosIng is the European Commission database for information on cosmetic substances and ingredients contained in the: Cosmetics Regulation (EC) No 1223/2009 of the European Parliament and the Council; Cosmetics Directive 76/768/EEC (Cosmetics Directive), as amended; Inventory of Cosmetic Ingredients as amended by Decision 2006/257/EC establishing a common nomenclature of ingredients employed for labeling cosmetic products throughout the EU. Opinions on cosmetic ingredients of the Scientific Committee for Consumer Safety (List of SCCS opinions published on the internet).
All cosmetic ingredient lists must use INCI (International Nomenclature of Cosmetic Ingredients) names for all cosmetic ingredients. The use of trade or common names is not allowed on cosmetic ingredient lists. INCI names are uniform scientific names. INCI names are mandated on the ingredient statement of every consumer personal care product.
Surfactants
Table of Contents
Surfactants are compounds that lower surface tension or interfacial tension. Reducing the surface tension allows a liquid to spread easier while reducing the interfacial tension (interfacial meaning between two faces) between water and an oil/lipid allows them to mix. A surfactant is composed of two parts, a hydrophilic head and a hydrophobic tail that are important determinants of lowering the surface tension. When in water, the hydrophilic head faces the water and the hydrophobic tail occupies minimal volume away from water to produce micelles (Fig.1), lowering the surface tension (superficial free energy).
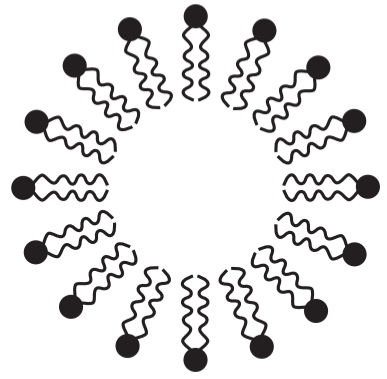
*Note: This is for oil-in-water, for water-in-oil, it is reversed where the tails are the outside and the heads are inside due to polarity.
The concentration of a surfactant at which micelles are formed is designated as Critical Micelle Concentration. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, and dispersants.
Common Types of Surfactants in Cosmetic Products:
- Acyl-amino acids and salts
- Alkyl ether sulfates
- Alkyl glyceride sulfates
- Alkyl polyglucosides or glycerides
- Alkyl sulfates
- Alkyl sulphonates
- Alkyl-aryl sulphonates
- Amide ether sulfates
- Betaines
- Carboxylates
- Decyl glucoside
- Disodium cocoamphodiacetate
- Ethanolamides or alkanolamides
- Fatty acid isethionates
- Phosphate esters
- Polyglucose/lactylate blend
- Sulfates
- Sulphoacetates
- Sulphonates
- Sulphosuccinates
- Taurates or Taurids
Types of Surfactants
Surfactants are classified based on polar heads. A non-ionic surfactant has no charged groups in its head (a). If the head carries a negative charge, then it is anionic (b). If it has a positive charge, then it is classified as cationic (c). If a surfactant contains a head with two oppositely charged groups, it is termed zwitterionic or amphoteric (d) [See Fig.2]. The question remains, which surfactants are harsh to skin and hair and which ones are milder and hence do not strip off our hair and are less irritating to the skin.
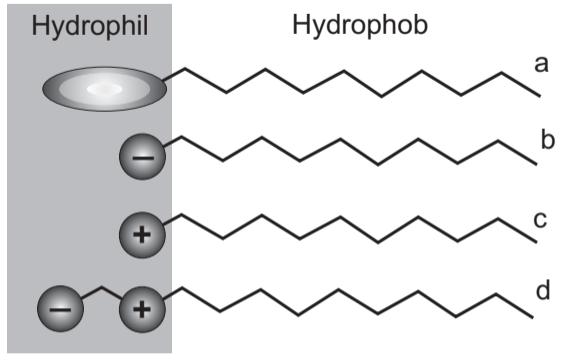
(A) Anionic Surfactant:
Most of the available shampoos contain one of the anionic surfactants given below, as their primary surfactant. Following sulfates are the harshest anionic surfactant(s) that should be avoided.
- Sodium Lauryl Sulphate (SLS)
- Sodium Lauryl Ether Sulphate (SLES)
- Ammonium Lauryl Sulphate
- Ammonium Laureth Sulphate
- Sodium Myristyl Sulphate
- Sodium Myreth Sulphate
- Sodium Coco or Cocoyl Sulphate
- Sodium C14-16 Olefin Sulphonate
- TEA Lauryl Sulphate
- TEA-dodecylbenzenesulphonate
- Sodium Alkylbenzene Sulphonate
- Ammonium or Sodium Xylenesulphonate
(B) Milder and Gentle Anionic Surfactants are:
- Sodium Cocyl Isethionate
- Sodium Lauryl Sulphoacetate
- Sodium Socoyl Sarcosinate
- Ethyl PEG-15 Cocamine Sulphate
- Dioctyl Sodium Sulphosuccinate
- Sodium Lauryl Glucose Carboxylate
- Sodium Methyl Cocoyl or Lauryl Taurate
- Sodium Cocoyl Glycinate
- Sodium lauroyl/cocoyl glutamate
(C) Cationic Surfactants
As opposed to other classes of surfactants, these positively charged surfactants are used primarily in conditioners. This class of surfactants is well known as a conditioning agent. Our hair is negatively charged. The more the hair is damaged, the more negative charge it carries. So, these positive charged cationic surfactants bond to the negatively charged hair and get adsorbed (not absorbed) on the hair shaft, and provide the conditioning effect.
The most common cationic surfactants are:
- Cetrimonium chloride
- Cetrimonium bromide
- Behentrimonium methosulphate
- Behentrimonium chloride
- Quaternium-15
- Quaternium-18
- Quaternium-22
- Stearalkonium chloride
- Stearamidopropyl dimethylamine (lactate, citrate, propionate)
- Isostearamidopropyl dimethylamine
- Behenamidopropyl dimethylamine
(D) Non-Ionic Surfactants
These are gentler than anionic surfactants and have inferior foaming properties than anionic surfactants. These are generally combined with other anionic surfactants to create milder shampoo.
Non-ionic surfactants are:
- Decyl glucoside
- Laureth (10, 23, 4)
- PEG-10 Sorbitan Laurate
- Polysorbate (20, 21, 40, 60, 61, 65, 80, 81)
- Steareth- (2, 10, 15, 20)
- Cocamidopropylamine oxide
(E) Amphoteric (or Zwitterionic) Surfactant
These surfactants provide mild cleansing and are added with other anionic surfactants to reduce the irritation caused by them. They help to boost foam formation when combined with other surfactants. These surfactants cannot be used alone in the products as these do not provide good cleansing properties, so are always to be used as secondary surfactants.
Amphoteric surfactants are:
- Cocamidopropyl betaine
- Coco betaine
- Sodium Cocoamphoacetate
- Disodium cocoamphodiacetate
- Disodium cocoamphodipropionate
- Sodium Lauroamphoacetate
Surfactant in Shampoos and Conditioners
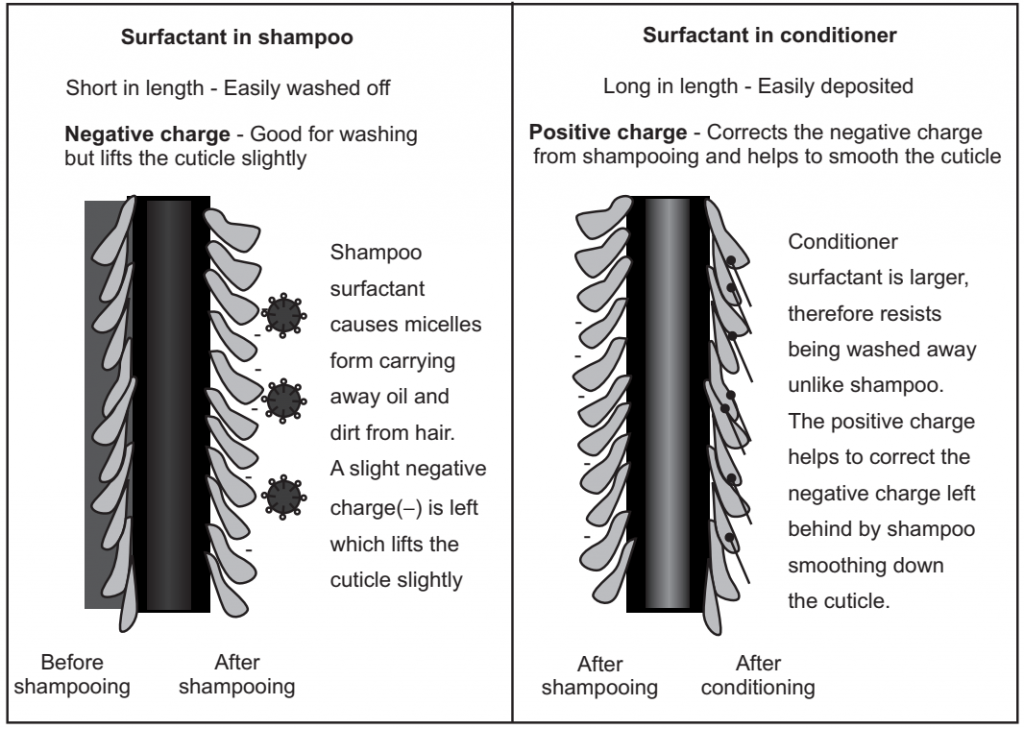
Every shampoo in the market whether it is a normal shampoo or one which claims to be sulfate-free always contains some kind of surfactant. Surfactants are added in the formulation for their cleaning property as they act as a detergent. Their main aim is to remove the dirt, excess sebum, and oil from the hair. Curly hairs are already moisture-deprived so using the shampoo containing harsh surfactants may lead to the stripping off of natural oils and moisture from the hair.
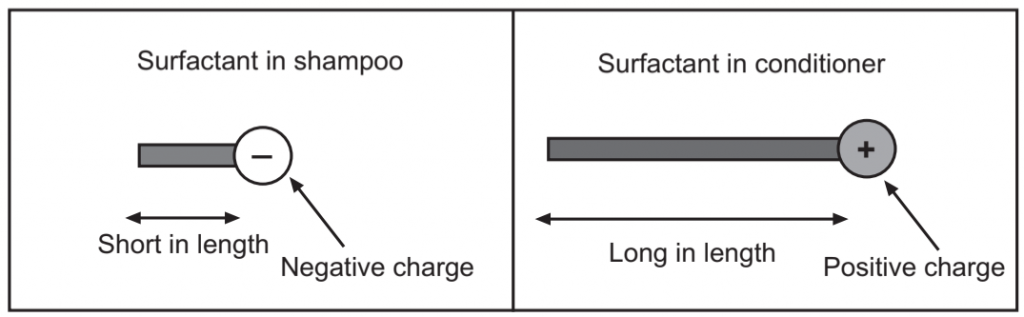
Five main applications of surfactants include Cleansing, Emulsification, Solubilization, Conditioning, and Special effects. Surfactant in shampoo is intended to clean and the one found in conditioner is intended to deposit. The key difference between the two types is
- Size or Length (Fig.3).
- Charge (Negative in shampoo and Positive in conditioner) (Fig.4).
Shampoos have surfactants like SLS, SLES, ALS and their purpose is to remove oil and wash hair. Conditioners have surfactants like behentrimonium chloride, behentrimonium methosulphate, and stearamidopropropyl which are designed to deposit onto hair, smooth the cuticle and create softness. While conditioner always has large positively charged surfactants, shampoo does not always have a negative small surfactant. Some sulfate-free shampoos utilize surfactants that are larger and have no charge or have a variable charge depending on pH.
Rheology Modifiers
The development of personal care formulations will necessarily include the consideration of the required product rheology and the correct rheology modifier to provide these effects. Whilst the efficacy of the finished product will depend on the choice and level of active ingredients and excipients, the aesthetics and even the penetration of these actives into the stratum corneum will be affected by the product rheology. Rheology can be defined as “the science or study of how things flow”, and it is a requirement of personal care products that they flow in the right way. A skin cream should appear highly viscous in the jar but should be capable of being picked up and rubbed into the skin. Nail polish should be sufficiently viscous to suspend the high volume of suspended pigment, not drip from the brush but thin sufficiently on the nails to provide even coverage without any obvious brush strokes.
The terms “rheology modifier”, “thickeners” and “gelling agents” appear to be the same, but these have different functionalities as listed in the table.1 below.
Table.1
| Thickening agents | Gelling agents | Rheology modifiers |
| Thickening Agents are added to increase the viscosity of a formulation (e.g. emulsion) without any other significant change to the performance of emulsion behavior. Some thickeners will help to stabilize your emulsions while de-stabilize. Almost none of these are waxes, though they are usually in solid form. | Gelling Agents are ingredients that turn the water, or oil phase into a gel, which is thickened, but without stiffness. The gels may be thixotropic. These gels make it possible to create thick products, which can be shaken, or stirred under high shear, for easier bottling, or spraying. | Rheology Modifiers are ingredients that alter the flow of a product. Additionally, they also alter the feel, offering increased slip and silkiness, and behavior. They usually improve the suspension capability of any gel while also creating a more fluid product that flows easily. Most rheology modifiers will also improve absorption rates, and depth, while eliminating dryness. |
Rheology modifiers are often referred to as thickeners, and whilst increasing the apparent viscosity will confer a feeling of “quality” to the formulation, this is only one aspect of rheological control. Rheology modifiers serve the purpose of not just altering the viscosity of the formulation but also that providing specific functionality to the product. This could range from improving body, texture, moisture retention, and dependability of soluble ingredients to increasing stability and dry strength, inhibiting syneresis, resisting bacterial attack, and preventing shrinkage. The product itself can be Newtonian or pseudoplastic, thixotropic, be a gel, or a flowable liquid. This will affect the way a product appears in the bottle, how easy it is to pour or scoop from the packaging, how easy it is to rub into the skin or along the hair shaft, and how easy it is to rinse and remove the product after use. The correct rheological characteristics are significant to ensure the stability of the finished formulation. To achieve such varied effects, different types of rheology modifiers are available to the formulator. These include inorganic and organic rheology modifiers. The latter is further classified into natural and synthetic (Fig.5).
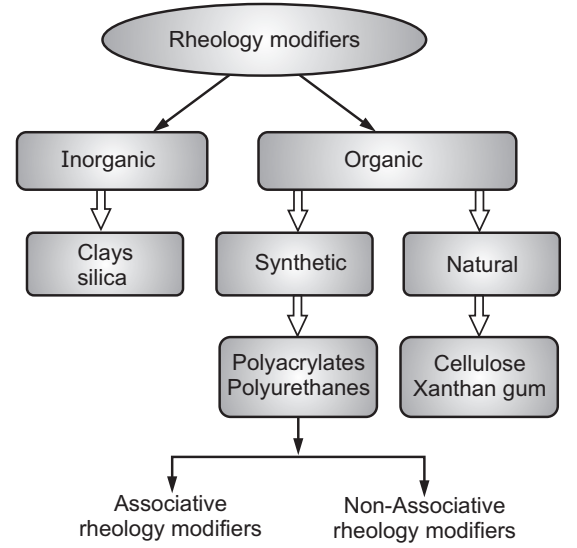
Naturally occurring polymers comprise polysaccharide or amino acid building blocks and are generally water-soluble. Common examples are starch, cellulose, alginate, egg yolk, agar, arrowroot, carrageenan, collagen, gelatin, guar gum, pectin, and xanthan gum. On the other hand, synthetic acrylic-based polymers are conveniently grouped into three general classes:
- Alkali-swellable (or soluble) emulsions (ASE’s)
- Hydrophobically modified alkali-swellable emulsions (HASE’s), and
- Hydrophobically modified ethoxylated urethane resins (HEUR’s).
HASE’s are modifications of ASE’s following the addition of hydrophobic functional groups. These are commonly known as associative thickeners. In its simplest form, an associative thickener is a water-soluble polymer containing several relatively hydrophobic groups. Heuer’s also belongs to the category of associative thickeners. But unlike HASE’s, HEUR’s are non-ionic substances and are not dependent on alkali for activation of the thickening mechanism. Typically in the form of white, fluffy, dry powder, popular varieties of synthetic thickeners include carbomers, sodium carboxymethylcellulose (CMC), and fumed silica.
Mechanism of Action
Rheology modifiers alter a system’s viscosity through a combination of mechanisms. Addition of alkali to an emulsion consisting of tightly coiled polymers that generate anionic charges along the chains. Like charges repel each other and the polymers swell and uncoil occupying more volume within the solution. Also, hydrophobic groups form domains along with other water-hating groups, ultimately reducing overall free energy and manifesting as a more structured, less fluid system. Lastly, particle-to-particle interactions between thickener and charged surfaces of system components bring about changes in rheological properties. The ease, by which thickeners are effectively dispersed or dissolved in the solvent, chemically depends on particle size, molecular weight, and structure (average number and distribution of hydroxyl groups per compound), and also the presence of a surfactant.
Specifically, the Rheology modifiers may present two predominant mechanisms of thickening.
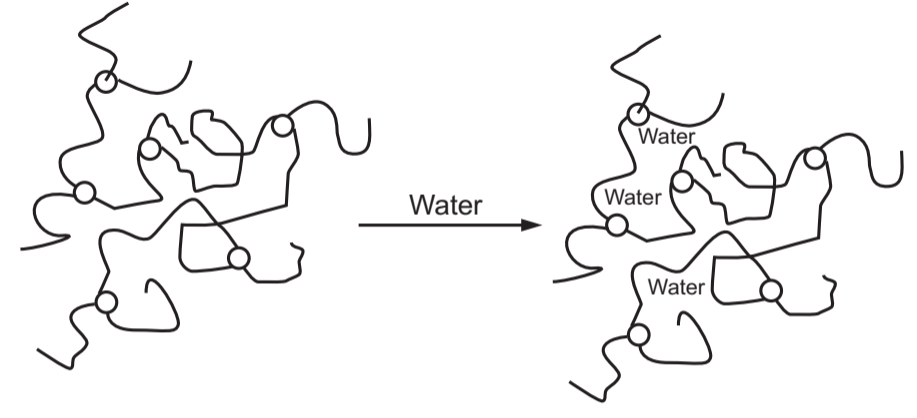
Volume Exclusion Thickeners employ water-soluble polymers that swell with water. Cellulose ethers, hydroxyethylcellulose, fall into this category. These types of thickeners create viscosity through chain entanglement (Fig. 3.6) and particle flocculation. Their ability to thicken is directly proportional to their molecular weight and concentration in the formulation. The greater the molecular weight, the more efficiently they get thicken.
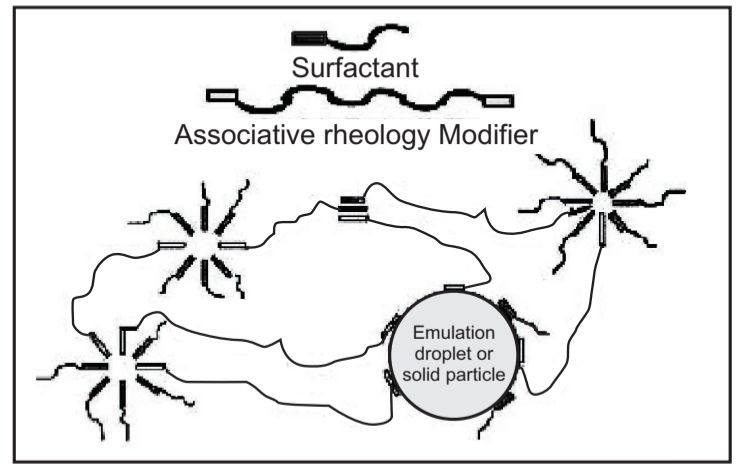
Associative Thickeners also employ water-soluble polymers, but they do not purely thicken through water absorption. Instead, these polymers contain hydrophobic groups that interact with each other and with the particles in the formulation to create a dynamic, reversible three-dimensional network. Thickening results because this network restricts the motion of the particles (Fig.7). Associative thickening can produce rheology ranging from Newtonian to pseudoplastic.
Non-associative thickening is thickening by an entanglement of water-soluble, high molecular weight polymer chains. The effectiveness of a thickener is mainly determined by the molecular weight of the polymer. Formulations thickened non-associatively have pseudoplastic rheology with highly elastic properties. This produces good stabilization against settling out and low sagging even with high build coatings. Non-associatively thickened systems often have limited flowability. The high molecular weight of the thickeners can sometimes lead to compatibility problems such as depletion flocculation.
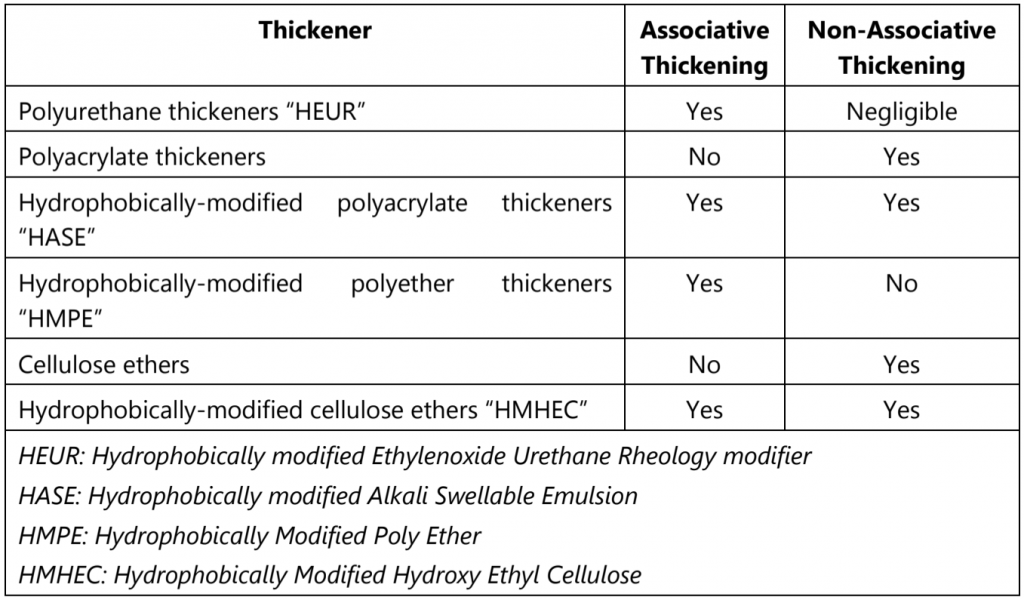
Table.2 presents an overview of different classes of thickener and thickening mechanisms.
Table.2
Applications
The applications of rheology modifiers can be readily understood based on the classification presented in Table.3 below:
Table.3
| Class | Examples |
| Lipid thickeners | Beeswax, cetyl alcohol, stearic acid, carnauba wax |
| Naturally derived thickeners | Modified cellulose, guar gum, xanthan gum, gelatin |
| Synthetic thickeners | Carbomers, polyethylene glycol |
| Ionic thickeners | Salts |
| Mineral thickeners | Silica, Bentonite |
(a) Lipid Thickeners
Lipid thickeners are primarily composed of lipophilic materials. They work by imparting their natural thickness to the formulation. Typically, these materials are solids at room temperature but are liquified via heat and incorporated into emulsions. They are used most often in creams and lotions.
(b) Naturally derived Thickeners
Various thickeners are found in nature or are derivatives of natural thickeners. These ingredients are polymers that work by absorbing water to swell up and increase viscosity. Cellulose derivatives like Hydroxyethylcellulose are frequently used in liquid cleansing products such as shampoo or body wash. Guar gum is another example of a naturally derived thickener. Others include Locust Bean Gum, Xanthan Gum, and Gelatin. These thickeners can be used in any formulation that contains a high level of water. Unfortunately, they can be inconsistent, cause clear formulae to become cloudy, and feel sticky on the skin.
(c) Mineral Thickeners
Mineral thickeners are naturally occurring, mined ingredients that can absorb water or oils and increase viscosity. Materials include Silica, Bentonite, and Magnesium Aluminium Silicate. These thickeners can be used to thicken oils as well as water-based formulations.
(d) Synthetic Thickeners
Perhaps the most versatile of all thickeners are synthetic molecules. Carbomer is the most common example. It is a water-swellable acrylic acid polymer that can be used to form crystal clear gels. They have a desirable feel which makes them superior to other thickening agents that leave a sticky feel. Carbomer thickeners also can suspend materials in solution so you can have low viscosity formulae with large particles suspended. These thickeners also help to stabilize emulsions and are frequently used in lotion and cream products.
(e) Ionic Thickening
Simply adding salt (NaCl) to a formulation containing anionic surfactant solution can thicken the formulation. Salt is frequently used as an adjusting agent during production.
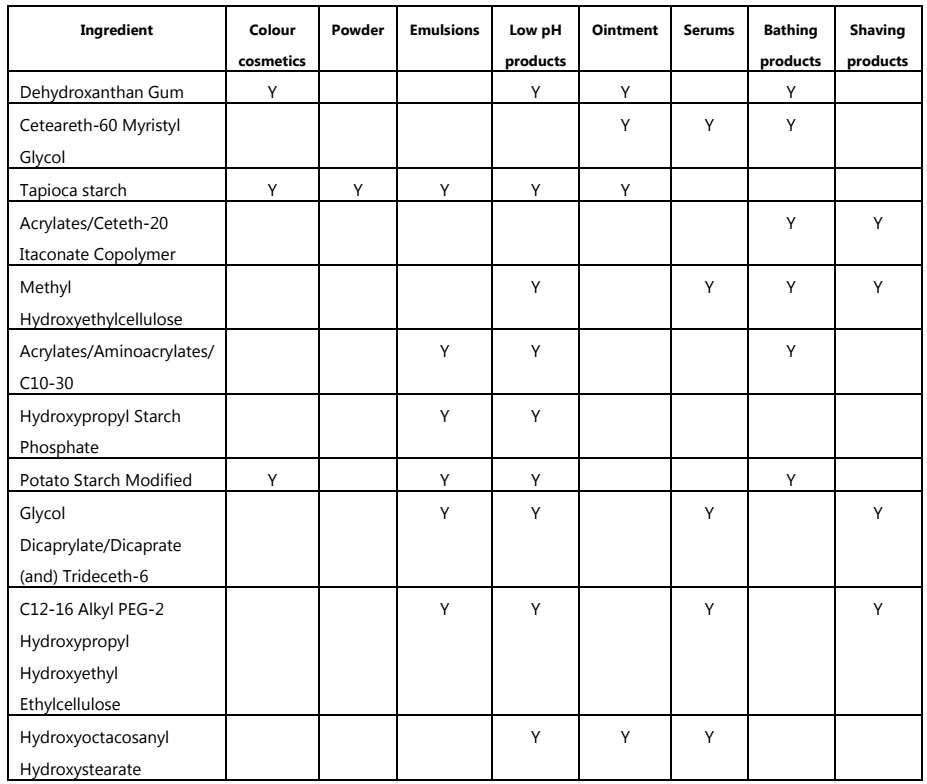
A cross-sectional list of rheology modifiers used in various cosmetic preparations is given in Table.4.
Table.4
Preservatives
Ideal cosmetic preservatives would have broad-spectrum activity, which is the ability of the preservative to kill a wide range of micro-organisms. Such a product would be effective against both Gram-negative and Gram-positive bacteria, yeast, mold, and fungus. Usually, a multiple preservative system, and /or the incorporation of hurdle technology, is needed to accomplish this goal. Preservatives should be effective at low concentrations to reduce costs, minimize toxicity effects, and not adversely affect the physical properties of the finished product. Preservatives should be stable under whatever conditions they may encounter in the manufacture of the finished product (i.e. temperature, pH, etc.). As it relates to all ingredients, but especially with preservatives, it is crucial that they are calculated, and measured, by their relative weight, not volume, to guarantee that adequate ratios are being used. All safety, and efficacy, studies, performed on the preservative, to determine usage ratios, are done in weight. Calculating, and measuring, by weight is the only reliable way.
Microbiological Considerations
The organisms that are capable of compromising both product integrity and safety are divided into three broad categories: bacteria, yeast, and mold. For optimum growth, bacteria require neutral or slightly alkaline pH and warm temperatures of 30-37o C. Bacteria can be divided into two classes based on a differential staining procedure, which distinguishes the chemistry in the bacterial cell wall. Gram-positive bacteria retain the primary stain while Gram-negative bacteria do not retain the stain materially. This distinction is very important because many of the Gram-negative bacteria are considered pathogenic or disease-producing while only a very few of the Gram-positive bacteria are considered pathogenic. Gram-negative bacteria are extremely difficult to control because of the complexity of their multilayered cell walls. The Gram-negative bacterium that is of greatest concern to formulators is Pseudomonas. Its species are widely distributed in nature and can be isolated from soil, tap water, marine water, and even from the skin. Pseudomonas can degrade a wide variety of organic compounds such as starch, cellulose, hydrocarbons, and resins. They are resistant to most antimicrobials and are often severe health hazards. P. aeruginosa is often responsible for wound infections, urinary tract infections, and severe eye infections, which can result in conjunctivitis or loss of sight.
Yeasts usually prefer an acidic pH and room temperature for optimal growth. They are of concern due to their effects on the aesthetics of the formulation than on health hazards. Candida albicans is the most common representative of this group. Mold, like yeast, also prefers an acidic pH and room temperature. They reproduce by spore formation and the spores can continue to survive indefinitely under favorable conditions. Spores are difficult to control as they can remain dormant in a hostile environment and can then become activated when the circumstances become conducive to their growth. A typical member of the group is Aspergillus niger, a widely distributed mold capable of product spoilage.
Micro-organisms, like most other living things, normally have three basic requirements that are essential for growth: water, air (for aerobes), and nutrients. Water is required to secure food and to eliminate waste products. However, even anhydrous or non-water-based products should be protected by preservative systems. During use conditions, a film of water can form on an anhydrous product, and in the high local concentration of water, microorganisms will grow.
Preservation in Cosmetic Formulations
Cosmetic formulations in particular are an excellent source of nutrients for microorganisms. Examples of ingredients that are especially good sources of food include: alcohols such as glycerol, sorbitol, mannitol, and fatty alcohols, fatty acids and their esters, sterols including lanolin and its derivatives, proteins, vitamins, and botanical extracts. Many variables influence the effectiveness of a preservative system. These include the concentration of the preservative, contact time, the number of micro-organisms present, protein kinase inactivation or enhancement by other ingredients in the formulation, and packaging.
In general, the higher the concentration of the preservative, the more effective it will be. Often preservatives are cidal that is they kill, at high concentrations and exhibit stasis, which prevents growth, at low concentrations. There is sometimes a tendency to want to “over preserve” products. This is not advised because high levels of preservatives activity are often associated with toxic or irritant properties on animal tissues. One must remember that preservatives are specifically designed to kill living cells. Therefore, at a particular concentration, these will undoubtedly affect the skin. On the other hand, too low a concentration of preservative may be ineffective or may even stimulate microbial growth.
The second factor is contact time between preservatives and the formulation. The longer the contact time, the greater would be the number of micro-organisms killed. Theoretically, micro-organisms are killed at a logarithmic rate. Under a specific set of conditions, the same percentage of a microbial population should be killed with each unit of time. The third factor regulating preservative activity is the number of micro-organisms challenging the system. The greater the number, the longer it would take for the preservative system to drop the count to some acceptable level.
The pH of the formulation is the fourth factor, as some preservatives, such as benzoic acid or parabens are only active in their acidic form. One must also consider the interaction between the preservative and other ingredients in the formulation. This interaction can result in either inactivation or enhancement of the preservative depending on the chemical reaction taking place. For example, anionic surfactants usually inactivate cationic preservatives. Proteins inactivate quarter series, parabens, and phenolics. Alternatively, enhancement of the preservative can occur when using raw materials such as alcohols, aldehydes, and acids because they often have some antimicrobial activity of their own. Another example of preservative enhancement is the use of EDTA (ethylenediaminetetraacetic acid) in a formulation. EDTA can be used to increase the permeability of the cell wall by chelating the metal ions that are part of its composition, thus increasing the organism’s sensitivity to a wide variety of preservatives. In addition, EDTA’s chelating ability allows it to chelate metal ions in the organism’s surroundings, depriving it of essential mineral nutrients.
A related problem is a potential interaction with packaging. The finished product packaging should be designed to prevent access of contaminants into the container, but also must be made of materials that will not cause inactivation of the preservative due to adsorption or complexation. Low-density polyethylene, for example, may adsorb parabens from a product.
The “Ideal” Preservative
1. An ideal cosmetic preservative would have broad-spectrum activity, which is the ability of the preservative to kill a wide range of micro-organisms. Such a product would be effective against both Gram-negative and Gram-positive bacteria, yeast, and mold. Usually, a multiple preservative system is needed to accomplish this goal.
2. A cosmetic preservative should be effective at low concentrations to reduce costs, minimize toxicity effects and not adversely affect the physical properties of the finished product.
3. Preservatives should be stable under whatever conditions they may encounter in the manufacture of the finished product (i.e. temperature, pH, etc.).
4. It should not affect either the color or the odor of the product and it should be compatible with the wide range of ingredients that may be found in the formulation.
5. The ideal cosmetic preservative should offer protection during manufacturing and should maintain activity throughout the intended life of the product in the hands of the consumer.
6. It should be easy to analyze in the finished product. This is more difficult than it sounds since determining the concentration present does not necessarily indicate that it is all available to preserve the product. For example, some of the preservatives may be bound to other chemicals or even the packaging, and therefore not be active.
7. The material should be easy to handle and safe for both the environment and humans. This is also not easy since a chemical that will kill micro-organisms is biologically toxic, and therefore has the potential to adversely affect the environment and mammalian tissue.
Types of Cosmetic Preservatives
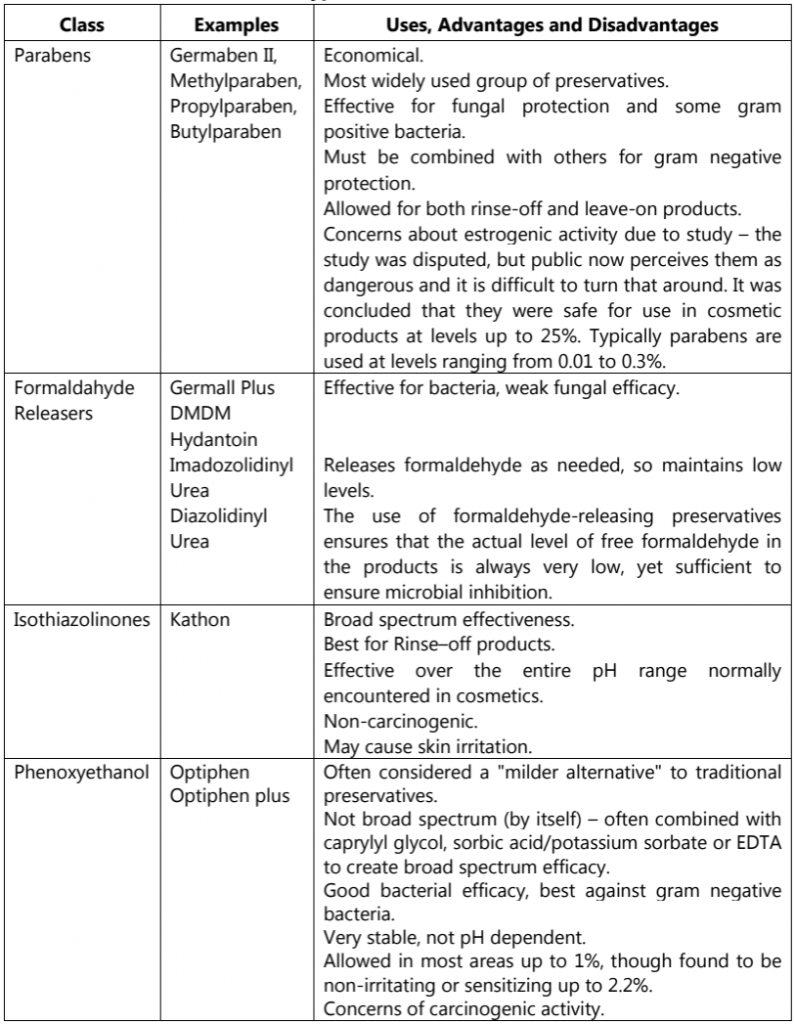
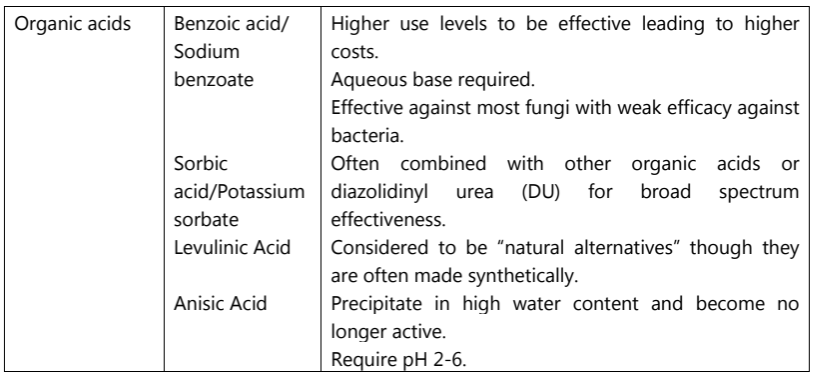
The five most common types of preservatives used in cosmetics, along with some examples, uses, advantages and disadvantages are listed below (Table.5) that can assist judicious selection of preservatives in cosmetic and personal care products.
Table.5: Types of Cosmetic Preservatives
Humectants
Humectants (or moisturizers) are important cosmetic ingredients that prevent loss of moisture thereby retaining the skin’s natural moisture. Some compounds also can actively attract moisture. Humectants are key ingredients in most skin care products but are also often used in hair care products to volumize the hair by attracting moisture that expands the hair shaft. There is a large variety of very different compounds providing moisturizing effects including proteins, acids, polysaccharides, and various small molecules (e.g. glycerine, sorbitol, urea, aloe vera, etc.). Humectants are common ingredients in a wide range of cosmetic and personal care products that make moisturization claims, namely hair conditioners, body lotions, face or body cleansers, lip balms, and eye creams.
Examples of some humectants include:
- Propylene glycol, hexylene glycol, and butylene glycol
- Aloe vera gel
- Alpha hydroxy acids such as lactic acid
- Egg yolk and egg white
- Glyceryl triacetate
- Honey
- Lithium chloride
- Molasses
- Polymeric polyols such as polydextrose
- Quillaia
- Sodium hexametaphosphate E452i
- Sugar alcohols (sugar polyols) such as glycerol, sorbitol, xylitol, maltitol
- Urea
Glycerin is one of the most popular humectants used because it produces the desired result fairly frequently and is low in cost. A category of humectants called nano lipid gels allows the skin to retain moisture but also possesses antifungal properties. Extracts from wine cakes also have the potential to be used as a humectant in cosmetics.
Humectants are frequently used in cosmetics as a way of increasing and maintaining moisture in the skin and hair, in products including shampoo, conditioner, frizz serum, lotions, creams, lip treatments, cleansers, after-sun lotion, and some soaps or body lotions. As hygroscopic moisturizers, humectants work by attracting water to the upper layer of the skin (stratum corneum). All humectants have hydroxyl groups which allow them to participate in hydrogen bonding and attract water. This process attracts moisture from the outer layer of the skin or, in high humidity, from the atmosphere. The moisture is then trapped against the epidermis or the shaft of the hair, depending on where the humectant is applied. Various humectants differ in water binding capacity at different humidities.
Humectants have been added to skin moisturizing products to treat xerosis. Some moisturizers tend to weaken the skin barrier function, but studies on xerosis have proven that moisturizers containing humectants increase desired moisturizing effects on the affected area without damage to the skin barrier function. However, “smarting and stinging” was reported from the use of humectant-rich treatment products. When glycerol is added to soaps for the cleansing of wounds, similar effects are seen. There is an increase in moisture in the areas where the soap was applied, but its healing property is uncertain.
Classification of Humectants
(A) Synthetic Humectants
Many of the humectants commonly used in skin care products withdraw water from the deeper levels of skin without replenishing it. This helps the surface of the skin to look more hydrated for a little while, but as it evaporates (which happens regularly unless in a very humid climate), it leaves the skin with less hydration overall, leading to dryness, dullness, and accelerated aging.
Skin care manufacturers prefer synthetic humectants because they cost less than natural ones. These will help to prevent water loss to some extent, but they provide no real nutrients to the skin, and over time, can cause damage. Some examples of synthetic humectants are as follows:
- Propylene glycol: A by-product of petroleum refining (and natural gas), propylene glycol can help skin hold onto moisture, but it can also dry out the lower layers of skin, contributing to future dullness, fine lines, and wrinkles. It can also be irritating to sensitive skin, causing redness and rashes.
- PEG: Polyethylene glycols (PEGs), like propylene glycol, are petroleum-based compounds that soften skin, but maybe contaminated with carcinogens like ethylene oxide and 1,4-dioxane. These withdraw moisture from the lower layers of skin but do not replenish it.
- Silicones: These are pervasive in today’s skin care products because they impart a smooth and soft feel on the skin, but these also form a film over the skin’s surface, keeping it from ‘breathing’ normally. Results may include increased acne and irritation, as well as future dryness.
- Urea: Urea is most often used as a preservative, but is also considered a humectant. It has been shown to release formaldehyde, a carcinogen, and can increase the risk of contact dermatitis.
(B) Natural Humectants
Natural humectants, not only help attract water to the surface of the skin, but also deliver hydration and nutrients to the deeper layers, as well. This helps the skin to keep itself hydrated regularly.
- Aloe: It is a wonderful humectant. It penetrates skin deeply and quickly, hydrating at the surface and the lower levels.
- Honey: Honey has a natural ability to hold onto water (the perfect humectant), hydrating without creating an oily feel. It is also a natural source of alpha-hydroxy acids, which encourages exfoliation. This makes it even easier for the skin to absorb the moisturizing elements.
- Hyaluronic acid: It is a natural molecule present throughout the body that helps hydrate and cushion joints, eyeballs, and skin. It has a natural ability to hold onto water and seems able to adjust according to humidity levels, helping your skin cope with even dry climates. This is an important ingredient in Anti-Aging serum, which helps the skin look firmer and tighter as well as reduces the appearance of fine lines and wrinkles.
- Glycerin: It occurs naturally in every living cell and is easily absorbed by the skin. It holds water well and it works by finding equilibrium between the water content in the air and the skin. Along with the benefits of deep hydration, the texture of glycerin makes it perfect for skin care because it glides on smoothly and evenly.
Humectants for Hairs
Humectants are added to hair care products because they attract water to the hair to keep the moisture content high. The examples of hair care humectants are given in the following Table.6.
Table.6
| 1,2,6 Hexanetriol Butylene Glycol Dipropylene glycol Glycerin Hexylene Glycol Panthenol Propylene glycol Sodium PCA Sorbitol Inositol Hexanediol beeswax Hexanetriol Beeswax Hydrolyzed Elastin Hydrolyzed Collagen Hydrolyzed Silk Hydrolyzed Keratin Erythritol Capryl glycol Isoceteth-(3-10, 20, 30) | Triethylene glycol Polyglyceryl sorbitol Glucose Fructose Polydextrose Potassium PCA Urea Hydrogenated Honey Hyaluronic Acid Isolaureth-(3-10, 20, 30) Laneth-(5-50) Laureth-(1-30) Steareth-(4-20) Trideceth-(5-50) Phytantriol enhances moisture retention, increases absorption of vitamins, panthenol, and amino acids into the hair shaft, imparts gloss |
Hair Penetrating Oils
Hair penetrating oils help to keep the hair moisturized in a two-fold process. These oils absorb into the hair preventing the loss of proteins that bind water molecules. This binding increases the amount of water that remains in the hair. They also minimize damage from expanding and contracting hair as water enters the hair shaft also known as hygral fatigue. As a result, the hair remains healthier during the shampooing process. Healthy hair stays moisturized. These oils are coconut, olive, and avocado oils. The opposite of penetrating oils is a coating or sealing of oils. These oils are impermeable to hair and are not moisturizers.
Anti Humectants
Anti-humectants create a thin invisible film on the hair that will repel water and moisture, keeping it styled.
Anti-humectant carrier oils
- Extra Virgin Olive Oil (EVOO)
- Coconut Oil (prefer unrefined)
- Waxes (such as beeswax, floral waxes, etc..)
- Mango Butter (prefer unrefined)
- Shea Butter (prefer unrefined)
Red Palm Oil (Its color is naturally in the red family. To avoid staining and the natural aroma many opt to use it refined. Refined has no color/ scent).
Synthetic Anti-Humectants
- Esters
- Silicones (prefer it to be water-soluble)
Emollients
The term emollient refers to materials that can soften skin. The word is derived from mollire which is a Latin verb meaning “to soften.” In the cosmetic formulating world emollients are ingredients incorporated into products to improve the feel of skin and hair. The use of these ingredients for cosmetic purposes; dates back to the earliest days of recorded history.
Traditionally, emollients are considered ingredients that have soothing or softening properties. They are added to the formulations to provide moisturizing benefits and support a variety of conditioning claims. Examples of emollients are ingredients like plant oils, mineral oil, shea butter, cocoa butter, petrolatum, and fatty acids (animal oils, including emu, mink, and lanolin, the latter probably the one ingredient that is most like our own skin’s oil). Others include triglycerides, benzoates, myristates, palmitates, and stearates, which are generally waxy in texture and appearance but provide most moisturizers with their elegant texture and feel. Emollients differ in their properties from humectants and occlusives. Table.7 given below describes the characteristics of each of the cosmetic ingredient categories.
Table.7
| Humectants | Emollients | Occlusives |
| Humectants function by attracting water outward to the SC from the dermis below and binding that water in the SC. e.g. Glycerin, urea, and pyrrolidone carboxylic acid (PCA). Glycerin is frequently used due to its low cost and high efficacy. However, the tackiness imparted to the skin by its high levels is one of the drawbacks. Thus, when optimizing skin formulations, cosmetic chemists often are challenged to reduce these negative properties. | Emollients provide some occlusivity and improve the appearance of the skin by smoothing flaky skin cells. Emollients generally are grouped by their ability to spread on the skin. By combining emollients with the different spread rates, formulators can tailor the skin feel of a moisturizer. Additionally, emollient lipids similar to those naturally found in the skin may also increase the rate of barrier repair. | Occlusive agents increase moisture levels in the skin by providing a physical barrier to epidermal water loss. Ingredients with occlusive properties include petrolatum, waxes, oils, and silicones. Some occlusive agents like petrolatum can leave a heavy feeling on the skin; thus they often are combined with other ingredients like emollients to improve consumer appeal. |
Classification of Emollients
Emollients are a class of ingredients with a wide variety of molecular structures. They are typically non-polar materials and come from both natural and synthetic sources.
(a) Hydrophilic Emollients
The term emollient is rather broad, so things that are humectants can also be considered as emollients. Water-soluble ingredients like glycerin, sorbitol, and propylene glycol are all technically emollients. These are good for conditioning in the water phase.
(b) Lipophilic Emollients
These are ingredients that are not soluble in water and make up the bulk of the available varieties of emollients. Judicious selection depends on properties such as polarity, emolliency scores, spreading behavior, compatibility with other ingredients, rheological behavior, and hydrolytic stability. This group can further be classified based on the polarity of emollients.
Non-polar: These are mostly derived from petroleum and include ingredients like mineral oil, isoparaffin, and isohexadecane.
Polar: This includes a range of ingredients including materials such as natural oils (jojoba oil, olive oil, coconut oil), esters (octyl palmitate, isopropyl stearate, isopropyl palmitate), and alcohols (octyl dodecanol).
(c) Silicone Fluid Emollients
They provide incredible levels of slickness and also feel lighter compared to lipophilic emollients. The most common ones used include Cyclomethicone and dimethicone. There are several varieties to choose from and each has different characteristics when it comes to viscosity, volatility, and ease of formulation.
Each of these ingredient types has a different mechanism of action and most cosmetic moisturizers will use a combination of them to create a synergistic effect and to mitigate certain esthetic or financial drawbacks. Product claims and skin feel are also considerations; therefore, experimentation with the various options is recommended. Table.8 below exemplifies emollients used in cosmetics for skin and hair care.
Table.8: Emollients used in cosmetics for skin and hair care
| Ingredient | Description |
| Triglycerides (Most common natural emollient from plant and animal sources) | Are made up of three fatty acids attached to glycerin. When from the plant, are referred to as oil: coconut oil, soyabean oil, palm oil. When from animal, are referred to as fat: Lard and tallow; exception shark oil and mink oil. Are used to derive cosmetic raw materials. The material is chemically modified to separate the fatty acids from the glycerine. The free fatty acids are used to create emollients called esters. |
| Lanolin (An emollient derived from sheep’s wool) | Is a more complex molecule than triglycerides. It is mostly non-polar and comprised of a complex mixture of esters. It is a soft solid at room temperature that melts at 40oC. Lanolin gives a nice feel to skin and hair. |
| Mineral oil | Most commonly used emollient. Is a complex blend of hydrocarbons, feels oily and greasy. |
| Emollient esters (Synthetic) (i) Simple esters (composed of an acid and an alcohol) (ii) Complex esters (Using molecules that have multiple acid or alcohol functional groups) (iii) Polyhydric alcohols (ethylene glycol) esters (Produced with molecules that have multiple alcohols (-OH) groups) | The fatty acids chemically removed from the triglycerides are reacted with various alcohols to produce emollients. E.g. Isopropyl myristate (reaction of isopropyl alcohol with myristic acid). A longer fatty acid will result in an ester with an oilier feel. E.g. Dioctyl sebacate and dioctyl maleate. Light feel and non-greasy. Used for lubrication; not adapted for cosmetics E.g. Ethylene Glycol Mono Stearate (EGMS). It is solid at room temperature and is used in formulations to produce a pearling effect. Ethylene glycol can be polymerized to produce very high molecular weight emollients that can be used as thickeners. PEG-150 distearate is an example of this. |
Make sure you also check our other amazing Article on : Cosmetics As OTC Drugs
