The aseptic area is the area where strict control measures are adopted to avoid contamination of the preparations or any transfer of microorganisms. Hence, proper care should be taken while designing the aseptic area. There are some differences between clean area, aseptic area, and sterile area which are listed in Table.1.
Table.1: Differences between Clean Area, Aseptic Area, and Sterile Area
| Clean | Aseptic | Sterile | |
| Procedure space | Onward | Dedicated area | Dedicated room |
| Gloves | Clean | Sterile | Sterile surgical |
| Hand hygiene before the procedures | Routine | Antiseptic (Alcohol) | Surgical scrub, Iodophores |
| Skin antisepsis | No | Alcohol | Long-acting agent |
| Sterile field | No | No | Yes |
| Sterile gown, mask, hand covers | No | No | Yes |
Building Design, Construction, and Production Facilities:
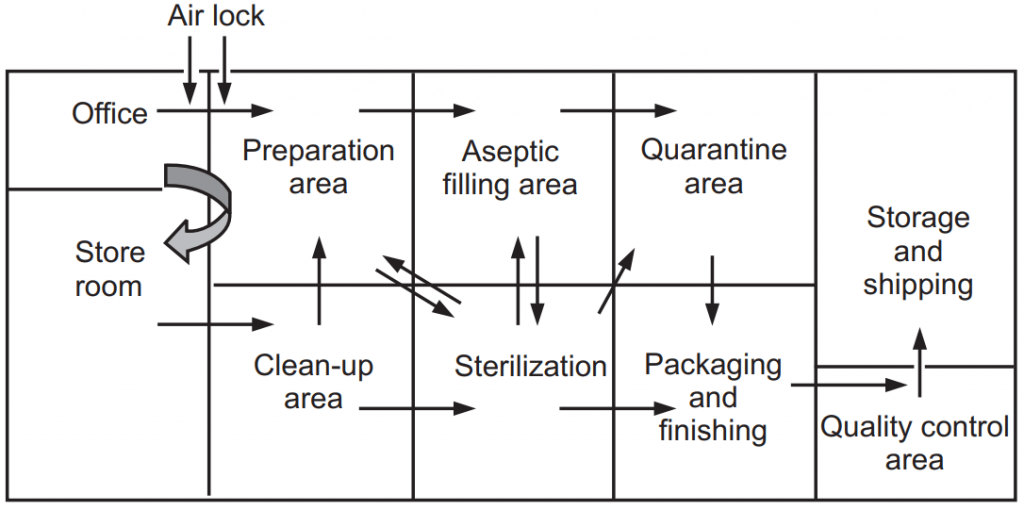
Production for sterile products is carried out in a clean environment. The environment should be freed from quality microbes and dust particle contamination. The limit of contamination is essential for reducing product contamination. The production area is having many other small areas like clean-up area, compounding area, aseptic area, quarantine area, and packaging area (Fig.1).
Floors, Walls, and Ceilings:
All the floors, walls, and ceilings must be smooth, easy to clean, disinfected, and be constructed to minimize microbial and particulate contamination. Flexing and non-flexing types of materials are used for the construction of the floor. Flexing materials are made up of synthetic elastomers like polyvinylchloride (PVC) are used for flooring which is easily repaired, cleaned, simple and cheap. Non-flexing flooring materials are made up of hard inorganic filler substances like the concrete floor is properly sealed with chemical resistant materials, solvents, and cleaning fluids.
Wall should be made up of non-inflammable materials like glass, stainless steel, etc. For minimization of fungal growth, 1% 8-hydroxyquinoline or pentachlorophenol is added with the paint for painting the wall. Epoxy resin paint, polyurethane paints are applied on the wall that helps to reduce the peeling of plaster and cracking of the wall. The ceilings are sealed to prevent the entry of contaminants. Cupboards, drawers, shelves, and other equipment are kept in an aseptic room as minimum as possible otherwise there is a chance for contamination.

Doors, Windows, and Other Services:
Doors and windows are fitted much closed together with the wall. Windows are kept at a minimum only for providing illumination; not for ventilation to minimize openings. Doors must be minimal, and well fitted by maintaining positive pressure airflow and self-closing. All pipes passing through the wall should be sealed properly and should be closely fitted. They should be cleaned easily. The gas cylinder should be avoided in the aseptic room. The gas connection should be through a pipeline where the cylinder be kept outside the area. Sinks and drains must be avoided inside the aseptic room.
All lights are fitted with the ceiling or inside the ceiling and covered with glass so that to avoid deposition of dust particles, other contaminants and also to avoid the disturbance of the airflow pattern inside the room. All the switches of the lights should be outside the aseptic room.
Personnel and Protective Clothing:
Contamination mainly comes from the skin surface of the operator of the room. Persons should be neat and clean and reliable to the selected work. They should be in good health, skilled in good manufacturing practices, and free from any dermatological diseases otherwise it increases microbial load inside the aseptic area. Borne contamination can be controlled by limiting the more number of persons in a clean area. They should also be skilled in aseptic techniques. They should wear sterile protective clothing like headwear, rubber or plastic gloves, non-fiber shedding face masks, and footwear. All the clothes should be designed to prevent contamination from the body. Clothes should be sterilized by moist heat sterilization or ethylene oxide sterilization. Each time when the persons are entering inside the aseptic room they should wear freshly sterile dresses (Fig.2).

Cleaning and Disinfection:
Disinfection and cleaning procedures are used for the removal of microbes and other contaminations. Alkaline detergents, ionic and non-ionic surfactants are used as cleaning agents. Various types of disinfectants are used to prevent the development of resistant strains of microorganisms. Different concentrations of quats, sodium hypochlorite, ethanol, and formaldehyde solutions are used as disinfectants inside the aseptic room. Chlorhexidine in 70% alcohol is mainly used as a skin disinfectant.
Training is required for staff who works in a cleanroom:
- They should know about microorganisms and controlling microbial contamination.
- They should know the entry and exit rules inside the aseptic room.
- They should get personal hygiene training.
- They should know the specific microbial risk associated with specific production tasks.
- Training must be effective and documented and regular wise review is required.
Air supply:
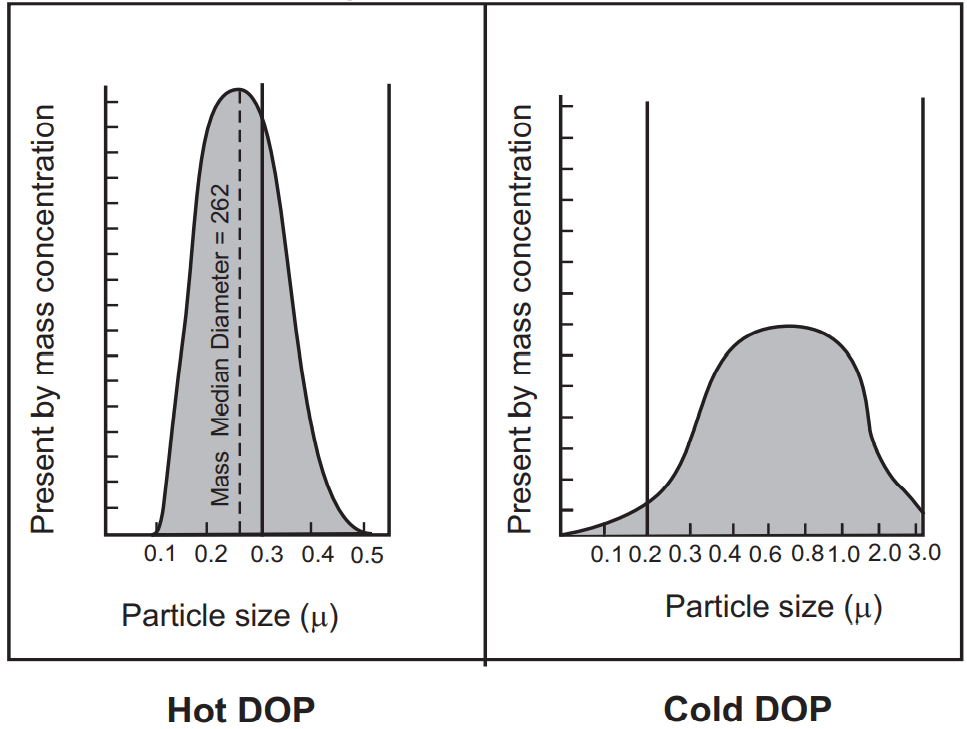
In the aseptic room, the air supply should be filtered through high-efficiency particulate air (HEPA) filter. The HEPA filter must be fitted at the inlet of the cleanroom and a prefilter also is fitted upstream of the HEPA filters to increase the life span of the original filters. HEPA filters are used in the construction of vertical and horizontal laminar airflow benches which filters 99.97% of the dust particles against 0.3 µm particles. HEPA filters are tested for efficiency by hot DOP (dioctyl phthalate) (efficiency test), cold DOP, and airflow resistance test. It is oil, used by air filter manufacturers and various testing agencies to make an aerosol to test the effectiveness of air filters. The DOP aerosol is used to challenge HEPA filters to test for efficiency. The hot DOP aerosol has a very narrow particle size distribution and is used to produce a high concentration of 0.3-micron particles that are considered the most penetrating of filter media. Cold DOP is having a broad particle size distribution aerosol which is useful for field testing for leaks and ensuring the integrity of an installation but does not provide the ultimate test of filter efficiency. The penetration or efficiency of a filter is strongly affected by the particle size of the challenge aerosol. A small change in particle size has a significant effect on penetration. The smaller the particle has the lower efficiency until the maximum penetrating particle size is reached (Fig.3).
Air Flow Resistance Test:
This method is a very fast and efficient measurement of HEPA filter. HEPA filters are supported to provide 100 air and they should be certified every 6-12 months. Air quality is evaluated by settle plates, microbial air sampler, and particle counters.
One more advanced technology used recently is ULPA. ULPA is Ultra-low penetration air. They are used where the smaller size particles are critical to their process mainly in semiconductor industries. These filters are replaceable extended media dry filters that have minimum particle collection efficiency of 99.9997% efficient for particles more than 0.12 microns in size. The ULPA filters are more costly than HEPA filters.
Make sure you also check our other amazing Article on : Antiseptics