Diabetes Mellitus Definition:
Table of Contents
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by a high blood glucose concentration-hyperglycemia.
- Hyperglycemia occurs because of uncontrolled hepatic glucose output and reduced uptake of glucose by skeletal muscle with reduced glycogen synthesis.
- When the renal threshold for glucose reabsorption is exceeded, glucose spills over into the urine (glycosuria) and causes an osmotic diuresis (polyuria), which, in turn, results in dehydration, thirst, and increased drinking (polydipsia).
- Insulin deficiency causes wasting through increased breakdown and reduced synthesis of proteins.
- Diabetic ketoacidosis is an acute emergency. It develops in the absence of insulin because of the accelerated breakdown of fat to acetyl-CoA, which, in the absence of aerobic carbohydrate metabolism, is converted to acetoacetate and β-hydroxybutyrate (which cause acidosis) and acetone (a ketone).
- Various complications develop as a consequence of the metabolic derangements in diabetes, often over many years. Many of these are the result of disease of blood vessels, either large (macrovascular disease) or small (microangiopathy).
- Dysfunction of the vascular endothelium is an early and critical event in the development of vascular complications. Oxygen-derived free radicals, protein kinase C and nonenzymic products of glucose and albumin (called advanced glycation end products) have been implicated.
- The macrovascular disease consists of accelerated atheroma and its thrombotic complications which are commoner and more severe in diabetic patients. Microangiopathy is a distinctive feature of diabetes mellitus and particularly affects the retina, kidney, and peripheral nerves.
- Diabetes mellitus is the commonest cause of chronic renal failure, which itself represents a huge and rapidly increasing problem, the costs of which to society as well as to individual patients are staggering.
- Coexistent hypertension promotes progressive renal damage, and treatment of hypertension slows the progression of diabetic nephropathy and reduces myocardial infarction.
- Angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists are more effective in preventing diabetic nephropathy than other antihypertensive drugs, perhaps because they prevent fibroproliferative actions of angiotensin II and aldosterone.
- Diabetic neuropathy is associated with the accumulation of osmotically active metabolites of glucose, produced by the action of aldose reductase, but aldose reductase inhibitors have been disappointing as therapeutic drugs.
- It is a lifelong metabolic disorder characterized by high blood sugar levels.
Diabetes Mellitus Symptoms:
- Hyperglycemia
- Blurred vision
- Fatigue
- Polyurea
- Polydipsia
- Polyphagia.
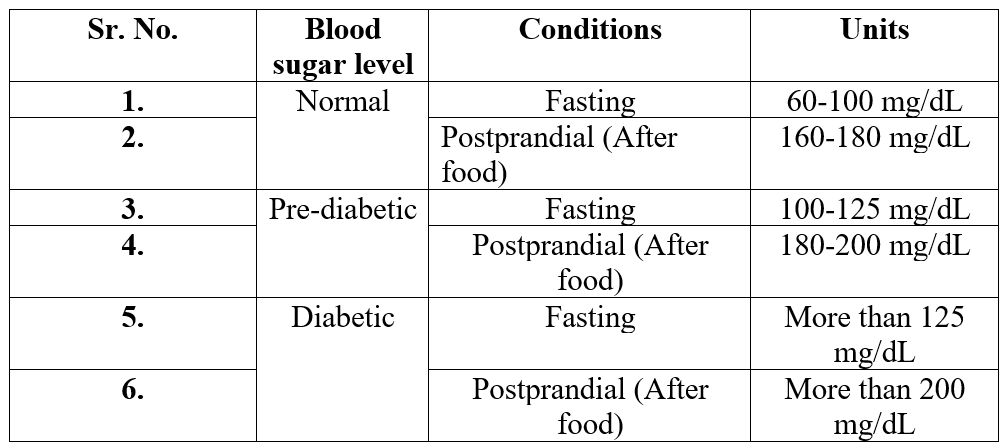
Blood sugar levels chart
Types of Diabetes Mellitus:
Type 1 Diabetes:
- Previously known as insulin-dependent diabetes mellitus-IDDM-or juvenile-onset diabetes.
- Generally, this occurs in patients below 20 years of age.
- Failure to produce Insulin.
- 15-20% of patients suffering from type 1 diabetes mellitus.
- In type 1 diabetes, there is an absolute deficiency of insulin resulting from autoimmune destruction of β cells. Without insulin treatment, such patients will ultimately die with diabetic ketoacidosis.
- Type 1 diabetic patients are usually young (children or adolescents) and not obese when they first develop symptoms. There is an inherited predisposition, with a 10- fold increased incidence in first-degree relatives of an index case, and strong associations with particular histocompatibility antigens (HLA types).
Type 2 Diabetes:
- Previously known as non-insulin-dependent diabetes mellitus-NIDDM-or maturity-onset diabetes.
- Generally, this occurs to patients over 40 years of age.
- Failure to utilize Insulin.
- 80-85% of patients suffering from type 2 diabetes mellitus.
- Type 2 diabetes is accompanied both by insulin resistance (which precedes overt disease) and by impaired insulin secretion, each of which is important in its pathogenesis. Such patients are often obese and usually present in adult life, the incidence rising progressively with age as β-cell function declines.
- Treatment is initially dietary, although oral hypoglycaemic drugs usually become necessary, and about one-third of patients ultimately require insulin. Prospective studies have demonstrated a relentless deterioration in diabetic control over the years.
Type 3 Diabetes:
- These types of diabetes occur due to other causes like chronic therapy with some drugs (Thiazide Urea, Glucocorticoids, Diazoxide, Growth Hormone) or disease-induced (Pancreatitis).
Type 4 Diabetes:
- This is also called gestational diabetes.
- 4-5% of patients suffering from type 4 diabetes.
- Increased blood sugar level than normal generally occurs during the third trimester and after postpartum period.
- The placental hormone promotes insulin resistance.
Diabetes Mellitus Treatment:
Classification:
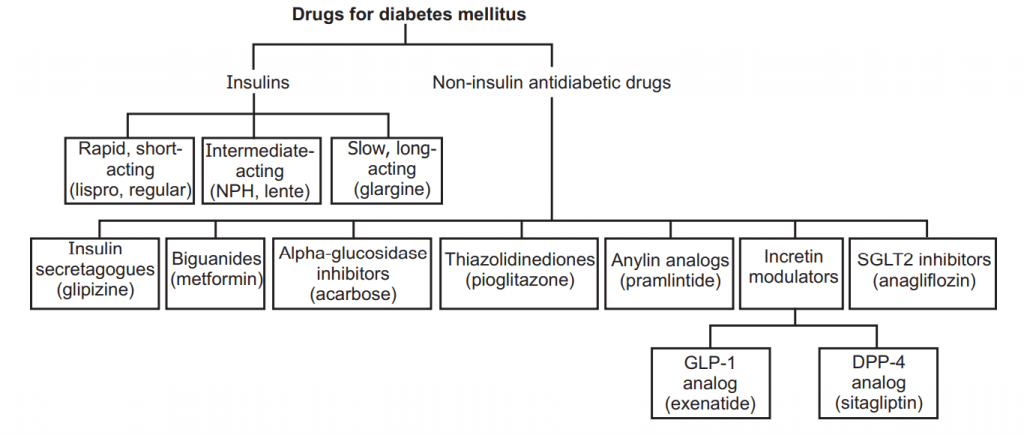
Anti-diabetic Drugs:
- Oral Hypoglycemic Drugs:
- Sulfonylurea Derivatives:
- First Generation: Tolbutamide, Chlorpropamide, Tolazomide, Acetohexamide.
- Second Generation: Glibenclamide, Glipizide, Gliclazide.
- Anti Hyperglycemic Drugs:
- Bioguanide: Phenformin, Metformin.
- Thiazolidine:Troglitazone, Coglitazone, Aeuglitazone, Pioglitazone.
- α- Glucosidase Inhibitors: Acarbose, Guargum, Miglitol.
- Parenteral Anti-diabetic Drugs: Insulin.
Oral Hypoglycemic Drugs
Sulfonyl Urea Derivatives:
Sulfonylurea:
- The sulfonylureas were developed following the chance observation that a sulfonamide derivative (used to treat typhoid) caused hypoglycemia.
- Numerous sulfonylureas are available. The first used therapeutically were tolbutamide and chlorpropamide. Chlorpropamide has a long duration of action and a substantial fraction is excreted in the urine. Consequently, it can cause severe hypoglycemia, especially in elderly patients in whom renal function declines inevitably but insidiously.
- It causes flushing after alcohol because of a disulfiram-like effect, and has an action like that of antidiuretic hormone on the distal nephron, giving rise to hyponatremia and water intoxication. Tolbutamide, however, remains useful.
- So-called second-generation sulfonylureas (e.g. glibenclamide, glipizide are more potent (on a milligram basis), but their maximum hypoglycemic effect is not greater and control of blood glucose not better than with tolbutamide.
- These drugs all contain the sulfonylurea moiety and act in the same way, but different substitutions result in differences in pharmacokinetics and hence in the duration of action.
MOA:
- The principal action of sulfonylureas is on β cells, stimulating insulin secretion and thus reducing blood glucose.
- High-affinity receptors for sulfonylureas are present on the KATP channels in β-cell plasma membranes, and the binding of various sulfonylureas parallels their potency in stimulating insulin release.
- These drugs reduce the permeability of K+ by competitively blocking sulfonylurea receptors present on ATP-sensitive K+ (KATP) channels.
- The inhibition of K+ channels causes the opening of voltage-gated Ca2+ channels, which leads to the increased influx of Ca2+ and thus stimulates insulin secretion from β cells of the pancreas.
- Reduced blood glucose level.
- They also suppress glucagon level which indirectly contributes to their hypoglycemic effects.
ADME:
- Sulfonylureas are well absorbed after oral administration, and most reach peak plasma concentrations within 2-4 hours. Glipizide absorption is delayed by the presence of food. The duration of action varies.
- All bind strongly to plasma (90-98%). Plasma protein bindings are less for first-generation drugs in comparison to the second generation. Metabolized in liver and kidney.
- Most sulfonylureas (or their active metabolites) are excreted in the urine, so their action is increased in the elderly and patients with renal disease.
- Most sulfonylureas cross the placenta and enter breast milk; as a result, the use of sulfonylureas is contraindicated in pregnancy and breastfeeding when diet and, if necessary, insulin is used.
Therapeutic uses:
- In type 2 diabetes mellitus.
- Surgery during diabetes.
- In diabetes coma.
ADR:
- Hypoglycemia may lead to renal and hepatic impairment.
- Weight gain, fluid retention, and edema.
- Photosensitivity, Skin Rashes, Blood dyscrasias, Cholestatic jaundice.
- Flatulence.
- It exhibits disulfiram-like action with alcohol due to inhibition of alcohol dehydrogenase causing accumulation of aldehyde dehydrogenase leading to headache, nausea, vomiting, and sweating.
Anti Hyperglycemic Drugs
Biguanide:
MOA:
- They increase glucose uptake and utilization in skeletal muscle (thereby reducing insulin resistance).
- Reduce hepatic and renal glucose gluconeogenesis, which reduced hepatic glucose outputs.
- Slowing down the glucose absorption from GIT, which increases the availability of glucose for its conversion to lactate by entrecote.
- It also promotes insulin binding to its receptor.
- Reduced plasma glucagon levels.
- It does not depend upon the functional state of β cells of the pancreas and can also be given to an obese person as it does not cause weight gain.
- While preventing hyperglycemia, does not cause hypoglycemia.
- Besides decreased elevated levels of glucose, it also decreases low-density and very-low-density lipoproteins (LDL and VLDL, respectively).
- All above action leads to Antidiabetic effects.
ADME:
- Well absorbs, distributed well throughout the body, metabolized in the liver, and excreted in unchanged form by the kidney.
- Metformin has a half-life of about 3 hours and is excreted unchanged in the urine.
Therapeutic use:
- In type 2 diabetes mellitus.
- In obese diabetes mellitus, as it does not stimulate appetite.
- Surgery during diabetes.
- In diabetes coma.
ADR:
- Nausea, Metallic taste, Anorexia, Flatulence, Diarrhoea, Gastrointestinal disturbances.
- Long-term use may interfere with the absorption of vitamin B12.
- Lactic acidosis is a rare but potentially fatal toxic effect, and metformin should not be given to patients with renal or hepatic disease, hypoxic pulmonary disease, heart failure, or shock.
- Such patients are predisposed to lactic acidosis because of reduced drug elimination or reduced tissue oxygenation. It should also be avoided in other situations that predispose to lactic acidosis and are contraindicated in pregnancy.
Thiazolidine:
MOA:
- Thiazolidine diones bind to a nuclear receptor called the peroxisome proliferator-activated receptor-γ (PPARγ), which is complexed with retinoid X receptor (RXR).
- PPARγ occurs mainly in adipose tissue, but also muscle and liver.
- It causes differentiation of adipocytes (this contributes to the unwanted effect of weight gain), increases lipogenesis, and enhances uptake of fatty acids and glucose.
- It also promotes amiloride-sensitive sodium ion reabsorption in renal collecting ducts, so causes fluid retention.
- Thiazolidinediones cause the PPARγ-RXR complex to bind to DNA, promoting the transcription of several genes with products that are important in insulin signaling.
- These include lipoprotein lipase, fatty acid transporter protein, adipocyte fatty acid-binding protein, Glut-4, phosphoenolpyruvate carboxykinase, malic enzyme, and others.
- It remains something of a mystery that glucose homeostasis should be so responsive to drugs that bind to receptors found mainly in fat cells.
- It also causes a reset of the glucose-fatty acid (Randle) cycle by the reduction in circulating free fatty acids.
ADME:
- Both rosiglitazone and pioglitazone are rapidly and nearly completely absorbed, with time to peak plasma concentration of fewer than 2 hours.
- Both are highly (> 99%) bound to plasma proteins, both are metabolized in the liver.
- Rosiglitazone is metabolized by CYP2C8 to weakly active metabolites, pioglitazone mainly by a CYP2C isozyme, and CYP3A4 to active metabolites. The metabolites of rosiglitazone are eliminated mainly in the urine and those of pioglitazone in bile.
Therapeutic use:
- In type 2 diabetes mellitus.
- In obese diabetes mellitus.
- Surgery during diabetes.
- Diabetes mellitus along with biguanides or sulfonylurea.
ADR:
- Weight gain, fluid retention, edema, subcutaneous accumulation of fats, hemodilution leading to a reduction in hemoglobin concentration, Hepatotoxicity.
α-Glucosidase Inhibitors:
MOA:
- Acarbose, an inhibitor of intestinal α-glucosidase, is used in type 2 patients whose diabetes is inadequately controlled by diet with or without other agents.
- It delays carbohydrate absorption, reducing the postprandial increase in blood glucose.
Carbohydrate (Polysaccharides)
↓ α glucosidase inhibitors
Inhibits Monosaccharide synthesis
↓
Slow down the absorption of monosaccharides from GIT → Anti-diabetic action
ADME:
- Well absorbed, distributed well throughout the body, metabolized in the liver, and excreted in unchanged form by the kidney.
ADR:
- Flatulence, Diarrhea, Abdominal pain, hypoglycemia (with sulfonylurea).
Insulin:
- Insulin was the first protein for which an amino acid sequence was determined (by Sanger’s group in Cambridge in 1955).
- It consists of two peptide chains (A and B, of 21 and 30 amino acid residues, respectively).
Synthesis and Secretion
- Like other peptide hormones, insulin is synthesized as a precursor (preproinsulin) in the rough endoplasmic reticulum.
- Preproinsulin is transported to the Golgi apparatus, where it undergoes proteolytic cleavage first to proinsulin and then to insulin plus a fragment of an uncertain function called C-peptide.
- Insulin and C-peptide are stored in granules in B cells and are normally secreted by exocytosis in equimolar amounts together with smaller and variable amounts of proinsulin.
- The main factor controlling the synthesis and secretion of insulin is the blood glucose concentration. β-cells respond both to the absolute glucose concentration and the rate of change of blood glucose.
- Other stimuli to insulin release include amino acids (particularly arginine and leucine), fatty acids, the parasympathetic nervous system, peptide hormones for the gut, and drugs that act on sulfonylurea receptors.
- There is a steady basal release of insulin and also a response to an increase in blood glucose. This response has two phases: an initial rapid phase reflecting release of the stored hormone, and a slower, delayed phase reflecting both continued release of the stored hormone and new synthesis.
Pre- pro-insulin (110 Amino acid)
↓ Endopeptidase
Pro-insulin (86 Amino acid)
↓ Protease in Golgi apparatus
Insulin (51 Amino acid, Chain A-21 and Chain B- 30 amino acids)
- Proinsulin (86 Amino acid, Chain A-21, Chain B- 30, and Chain C- contains 35 amino acids). Chain A and B are black and chain C is yellow.
- Human pancreas stores up to 8 mg of insulin which is equivalent to 220 units of insulin.
MOA:
- Insulin binds to a specific receptor on the surface of its target cells. The receptor is a large transmembrane glycoprotein complex belonging to the kinase-linked type 3 receptor superfamily and consisting of two α and two β subunits.
When Insulin binds to α subunits of the outer surface of the cells cause:
(a) Aggregation and internalization of Insulin receptors along with Insulin in vesicles, resulting in down-regulation, and activation of tyrosine kinase activity in β subunits.
(b) Result autophosphorylation of tyrosine kinase residue present on the cytoplasmic protein called Insulin receptor substrate I and Insulin receptor substrate II.
(c) This results in a cascade of phosphorylation and dephosphorylation reaction.
(d) Result stimulation or inhibition of enzyme system involved in rapid metabolism action of Insulin.
(e) Certain second messenger such as IP3 and DAG system activated and subsequent activation of Phospholipase-C.
Result in different actions like:
(a) Insulin stimulates glucose transport across the cell membrane by ATP-dependent transportation of glucose transporter-4 (GLUT-4), so uptake and utilization of glucose by the skeletal muscle are increased.
(b) Inhibits gluconeogenesis.
(c) Inhibits glycogenolysis.
(d) Stimulate glycogenesis.
(e) Stimulate glucogenesis.
(f) Stimulate storage of glycogen, fat and proteins.
- All these activities lead to decrease blood sugar levels, Antidiabetic action.
Pharmacological Action
Effect of Insulin on Carbohydrate Metabolism:
In Liver:
- Insulin influences glucose metabolism in most tissues, especially the liver, where it inhibits glycogenolysis (glycogen breakdown).
- It also inhibits gluconeogenesis (synthesis of glucose from non-carbohydrate sources) while stimulating glycogen synthesis.
- It also increases glucose utilization (glycolysis), but the overall effect is to increase hepatic glycogen stores.
In Muscles:
- In muscle, unlike the liver, uptake of glucose is slow and is the rate-limiting step in carbohydrate metabolism.
- The main effects of insulin are to increase facilitated transport of glucose via a transporter called GLUT-4 and to stimulate glycogen synthesis and glycolysis.
- Insulin increases glucose uptake by GLUT-4 in adipose tissue as well as in muscle, enhancing glucose metabolism.
- One of the main end products of glucose metabolism in adipose tissue is glycerol, which is esterified with fatty acids to form triglycerides, thereby affecting fat metabolism.
In adipose Tissue:
- Insulin increases the synthesis of fatty acid and triglyceride in adipose tissue and liver.
- It inhibits lipolysis, partly via dephosphorylation (and hence inactivation) of lipases.
- It also inhibits the lipolytic actions of adrenaline, growth hormone, and glucagon by opposing their actions on adenylate cyclase.
Effect of Insulin on Protein Metabolism:
In Liver:
- It inhibits the oxidation of amino acids in the liver.
- It also decreases the breakdown of protein in the liver.
In Muscles:
- Insulin stimulates the uptake of amino acids into muscle.
- Insulin increases protein synthesis.
Effect of Insulin on Fat Metabolism:
In Liver:
- Insulin increases Lipid synthesis (Lipogenesis)
In Adipose Tissue:
- It stimulates fatty acids synthesis and triglycerides formation
- Insulin inhibits Lipolysis
Other Metabolic Effects:
- Insulin increases the transport of K+, Ca2+, and Phosphate.
- Insulin stimulates vascular endothelial lipoprotein lipase activity and thus stimulates clearance of VLDL.
ADME:
- Being high molecular weight polypeptide insulin rapidly degraded in GIT if administered orally.
- Insulin can be administered by IV in an emergency condition, by IM, it absorbs more rapidly.
- Hence it is administered by SC, in which the rate of administration is slow and sustained action can be achieved.
- It is well absorbed and distributed well, metabolized in the liver by insulinase enzyme, and excreted in the urine.
Therapeutic Uses:
- Patients with type-I and type-II diabetes mellitus.
- For gestational Diabets mellitus.
- For emergency treatment of diabetic ketoacidosis.
- In acute alcoholism, Insulin, and glucose are given to hasten the metabolism of alcohol in the liver.
ADR:
- Hypoglycemia, Lipodystrophy (Site of injection).
- Allergic manifestation- Urticaria, Angeioedema.
- Blurred vision, obesity, nervous disorders, Very rarely anaphylaxis.
Management of Diabetic Coma:
- Glucose 5% by IV infusion.
- Glucagon 1 mg by IM route to raise sugar level.
- Diazoxide to treat resistance hypoglycemia, it causes the release of adrenaline and noradrenaline which increases blood sugar level.
- Insulin preparation.
Conventional (standard) preparation of insulin
| Types | Appearance | Onset (h) | Peak (h) | Duration (h) | Can be fixed with |
| Short-Acting | |||||
| Regular (crystalline solution) insulin | Clear | 0.5 | 2-4 | 6-8 | All Preparation |
| Prompt I. Tine suspension (Amorphous/semi lent) | Cloudy | 1 | 3-6 | 12-16 | Regular Lente preparation |
| Intermediate Acting | |||||
| Insulin tn. Suspension or Lente (ultra:semi;7:3) | Cloudy | 1-2 | 2-4 | 8-10 | Regular/semi Lente |
| Neutral protamine Hagedorn NPH OR isophane insulin tine. | Cloudy | 1-2 | 3-6 | 8-10 | Regular or crystalline |
| Long-Acting | |||||
| Extended I in suspension (crystalline form or ultra-Lente) | Cloudy | 4-6 | 14-18 | 24-36 | Regular and semi Lente |
| Protamine tine insulin (PTI) | Cloudy | 4-6 | 14-18 | 24-36 | Regular |
| Ultra-Short Acting | |||||
| Insulin-lispro | Clear solution | 10-20 | 1-2 | 3-4 | With all time of insulin |
| Insulin Aspart | Clear solution | do | do | do | do |
Glucagon:
Glucagon is a single-chain polypeptide of 21 amino acid residues.
Synthesis and Secretion
- Glucagon is synthesized mainly in the α cell of the islets, but also the upper gastrointestinal tract.
- It has considerable structural homology with other gastrointestinal tract hormones, including secretin, vasoactive intestinal peptide, and GIP. The endocrine pancreas and blood glucose.
- Islets of Langerhans secrete insulin from B (or β) cells, glucagon from α cells, and somatostatin from D/ delta cells.
- Many factors stimulate insulin secretion, but the main one is blood glucose.
- Insulin has essential metabolic actions as a fuel storage hormone and also affects cell growth and differentiation. It decreases blood glucose by:
(a) Increasing glucose uptake into muscle and fat via GLUT-4.
(b) Increasing glycogen synthesis.
(c) Decreasing gluconeogenesis.
(d) Decreasing glycogen breakdown.
(e) Glucagon is a fuel-mobilizing hormone, stimulating gluconeogenesis and glycogenolysis, also lipolysis, and proteolysis. It increases blood sugar and also increases the force of contraction of the heart.
- One of the main physiological stimuli to glucagon secretion is the concentration of amino acids, in particular L-arginine, in plasma.
- Therefore an increase in secretion follows ingestion of a high-protein meal, but compared with insulin there is relatively little change in plasma glucagon concentrations throughout the day.
- Glucagon secretion is stimulated by low and inhibited by high concentrations of glucose and fatty acids in the plasma.
- Sympathetic nerve activity and circulating adrenaline stimulate glucagon release via β adrenoceptors.
- Parasympathetic nerve activity also increases secretion, whereas somatostatin, released from D/delta cells adjacent to the glucagon-secreting α cells in the periphery of the islets, inhibits glucagon release.
Physiological Actions:
- Glucagon increases blood glucose and causes the breakdown of fat and protein. It acts on specific G-protein-coupled receptors to stimulate adenylate cyclase, and consequently, its actions are somewhat similar to β adrenoceptor-mediated actions of adrenaline.
- Unlike adrenaline, however, its metabolic effects are more pronounced than its cardiovascular actions. Glucagon is proportionately more active on the liver, while the metabolic actions of adrenaline are more pronounced on muscle and fat.
- Glucagon stimulates glycogen breakdown and gluconeogenesis and inhibits glycogen synthesis and glucose oxidation. Its metabolic actions on target tissues are thus the opposite of those of insulin.
- Glucagon increases the rate and force of contraction of the heart, although less markedly than adrenaline.
Therapeutic uses:
- Glucagon can be given intramuscularly or subcutaneously as well as intravenously.
- Treatment of hypoglycemia in unconscious patients (who cannot drink), unlike intravenous glucose, it can be administered by non-medical personnel (e.g. spouses or ambulance crew).
- It is useful if obtaining intravenous access is difficult.
- Treatment of acute cardiac failure precipitated by β-adrenoceptor antagonists.
Control of Blood Glucose:
- Glucose is the obligatory source of energy for the brain, and physiological control of blood glucose reflects the need to maintain adequate fuel supplies in the face of intermittent food intake and variable metabolic demands.
- More fuel is made available by feeding than is immediately required and excess calories are stored as glycogen or fat.
- During fasting, these energy stores need to be mobilized in a regulated manner.
- The most important regulatory hormone is insulin, the actions of which are described above. Increased blood sugar stimulates insulin secretion, whereas reduced blood sugar reduces insulin secretion.
- Hypoglycemia, caused by excessive insulin, not only reduces insulin secretion but also elicits secretion of an array of ‘counter-regulatory hormones, including glucagon, adrenaline, glucocorticoids and growth hormone, all of which increase blood glucose.
- Their main effects on glucose uptake and carbohydrate metabolism are summarised and contrasted with those of insulin.
Somatostatin
- Somatostatin is secreted by the D/delta cells of the islets.
- It is also generated in the hypothalamus, where it acts to inhibit the release of growth hormone. In the islet, it inhibits the release of insulin and glucagon.
- Octreotide is a long-acting analog of somatostatin.
- It inhibits the release of several hormones and is used clinically to relieve symptoms from several uncommon gastroenteropancreatic endocrine tumors and for treatment of acromegaly (the endocrine disorder caused by a functioning tumor of cells that secrete growth hormone from the anterior pituitary.
Amylin (Islet Amyloid Polypeptide)
- The term amyloid refers to amorphous protein deposits in different tissues that occur in a variety of diseases, including several neurodegenerative conditions.
- Amyloid deposits occur in the pancreas of patients with diabetes mellitus, although it is not known if this is functionally important. The major component of pancreatic amyloid is a 37-amino acid residue peptide known as islet amyloid polypeptide or amylin.
- This is stored with insulin in secretory granules in β-cells and is secreted with insulin. Amylin delays gastric emptying. Supraphysiological concentrations stimulate the breakdown of glycogen to lactate in striated muscle.
- Amylin also inhibits insulin secretion. It is structurally related to calcitonin and has weak calcitonin-like actions on calcium metabolism and osteoclast activity.
- It is also about 50% identical with calcitonin gene-related peptide (CGRP) and large intravenous doses cause vasodilatation, presumably by action on CGRP receptors.
- Whether amylin has a role in the physiological control of glucose metabolism is controversial, but there is interest in the therapeutic potential of amylin agonists (such as pramlintide, an analog with three proline substitutions that reduce its tendency to aggregate into insoluble fibrils.
Make sure you also check our other amazing Article on : Thyroid Hormones