Diasterioisomerism: Stereoisomers with two or more asymmetric or chiral carbons (stereocenter) will show Diasterioisomerism. The stereoisomers that are neither mirror images of one another nor are superimposable are known as diastereoisomers.
For example:
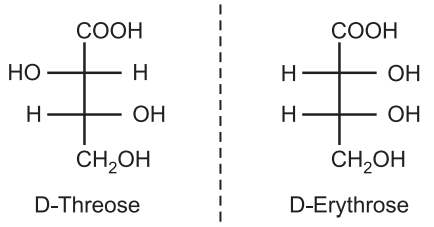
Each stereocenter gives two different configurations. It means if a molecule contains two asymmetric carbons, there are up to four possible conformations. When two diastereoisomers differ from each other at only one stereocenter they are known as epimers. e.g., D-threose and D-erythrose are epimers of each other. Unlike enantiomers, diastereoisomers have different physical and chemical properties.
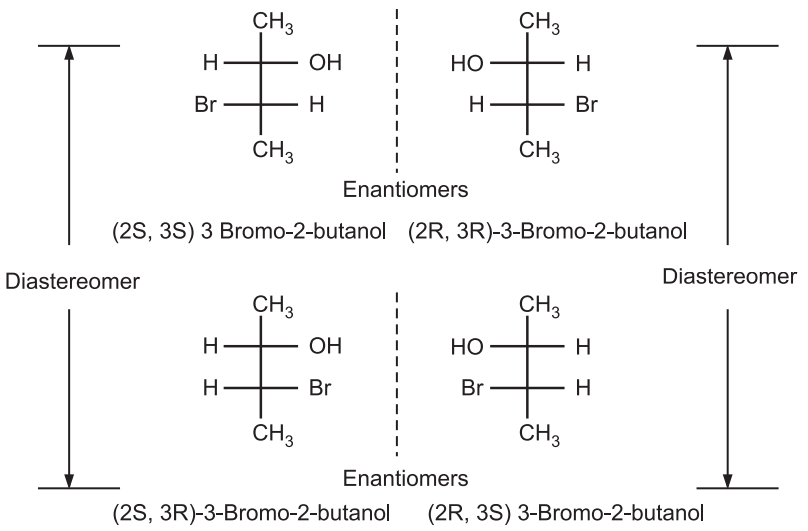
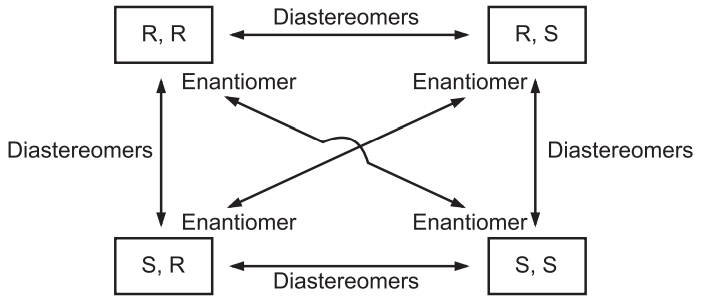
In the case of 3-Bromo-2-butanol, we have four possible combinations as SS, RR, SR, and RS. Out of these, two molecules SS and RR are enantiomers of each other while the configurations RS and SR are diastereomers of SS and RR configurations.
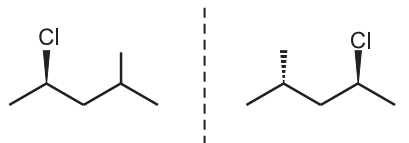
Thus in diastereoisomers, the chemical formula and atom connectivity remain the same but the three-dimensional orientation or shape of the molecule is different e.g., 2-Bromo-3- chloroethane.
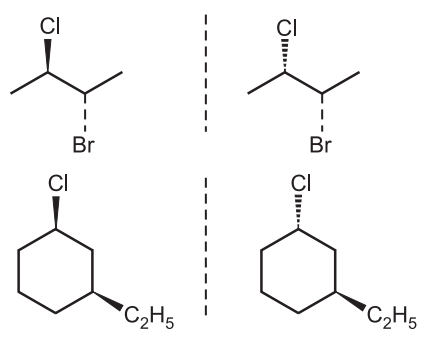
The molecules are different in the configuration of chlorine atoms but the same with the bromine atoms hence they are diastereomers. Similarly in cyclic compound 3-ethyl-1- chloro-cyclohexane, ethyl groups have the same configuration but the chlorine atoms have the opposite configuration. Hence, these molecules are diastereomers. Configurations differ at some stereocenters but not at others can not create mirror images. So they are not enantiomers but are diastereomers.
The dihydrotestosterone molecule contains seven stereocenters. Applying the 2N rule gives 128 possible configurations. Out of these, only one is enantiomeric pair while the rest are diastereomers.
Make sure you also check our other amazing Article on : Stereoisomerism