Elements of Symmetry: A chiral object is not identical (i.e. non-superimposable) in all respects. An achiral object is identical (hence superimposable) with its mirror image. Chiral objects have a “handedness”. Like gloves or shoes, chiral objects come in pairs, a right and a left. Achiral objects do not have a handedness just like a plain round ball. Thus, the chirality of an object is related to its symmetry. Certain symmetry elements like a point, a line, or a plane may be useful to check the symmetry of the molecule. The rotation or reflection around the symmetry element leaves the object in an orientation indistinguishable from the original. Reflection means the coincidence of atoms on one side of the plane with corresponding atoms on the other side, as though reflected in a mirror.
(i) Point of symmetry:
In a chiral molecule, (E)-1,2,-dichloroethene, two lines drawn passing through the point of symmetry ensure the same structural features at the opposite lines. Similarly, the boat conformation of cyclohexane has two intersecting planes of symmetry (σ). A plane of symmetry divides the object in such a way that the points on one side of the plane are equivalent to the points on the other side by reflection through the plane.
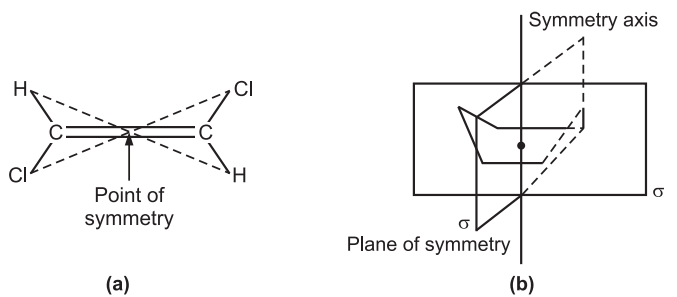
The existence of a reflective symmetry (a point or plane of symmetry) indirectly proves the molecule is achiral. Chiral molecules however may have rotational symmetry axes and do not have any reflective symmetry elements.
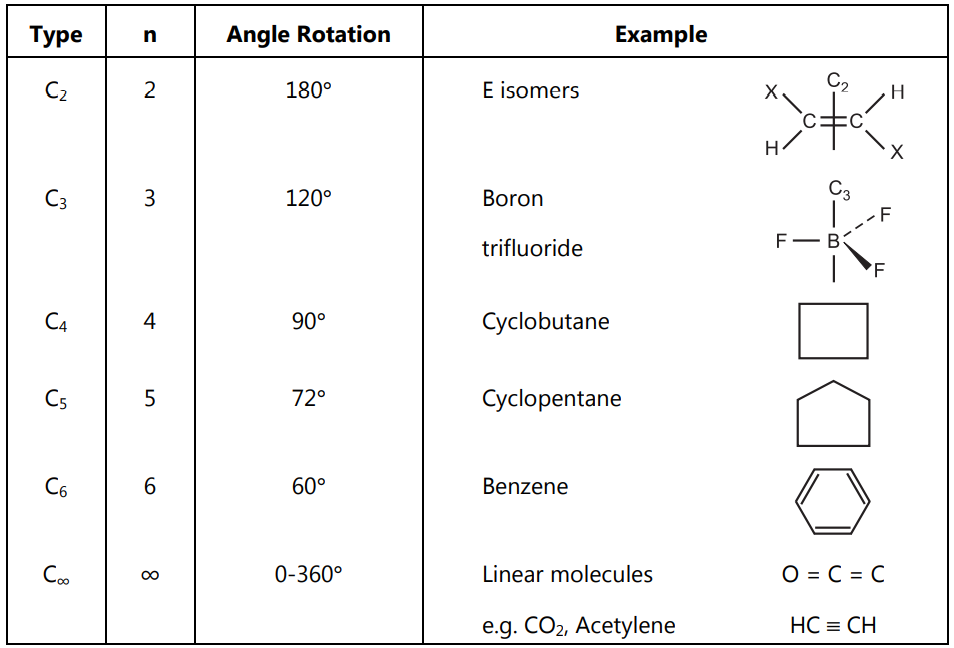
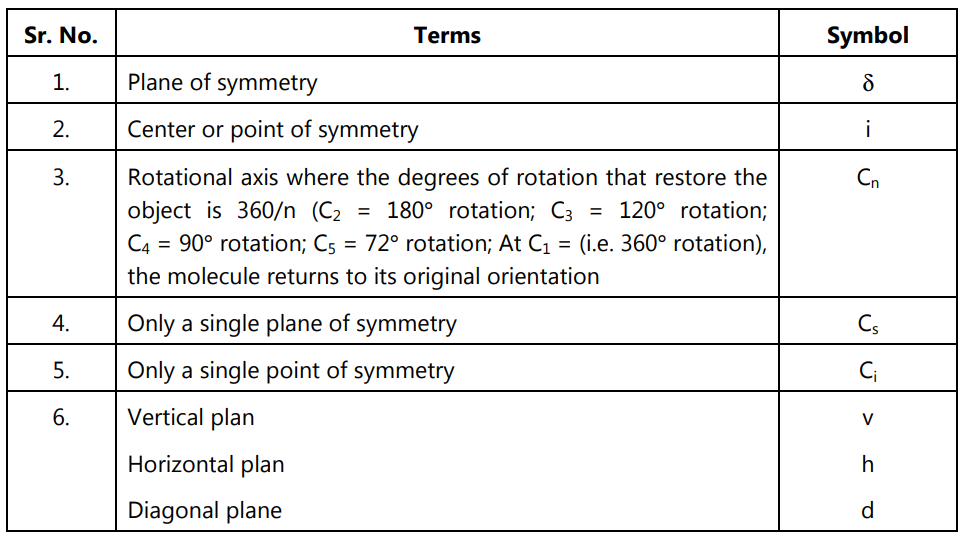
Examples:
(1) Methane: It is an example of a high symmetry molecule having 4 C3 axes, 3 C2 axes, and 6 σ (planes). It belongs to the tetrahedral point group Td. It is achiral.
(2) Cis-1,2-dichloroethane: This structure has two orthogonal planes of symmetry and the C2 axis at their intersection. It is achiral.
(3) Trans-1,2-dichloroethane: This structure has a plane of symmetry, an orthogonal C2 axis, and a point of symmetry at their intersection. It is achiral.
(4) Trans-1,2-dimethylcyclopropane: This structure has only a single C2 axis. It is dissymmetric and chiral.
(5) Cyclohexane (boat conformation): It has a C2 axis and two planes of symmetry. It is achiral.
(6) Cyclohexane (chair conformation): It has planes, axes, and a point of symmetry. The principal axis is C3.
(ii) Plane of symmetry:
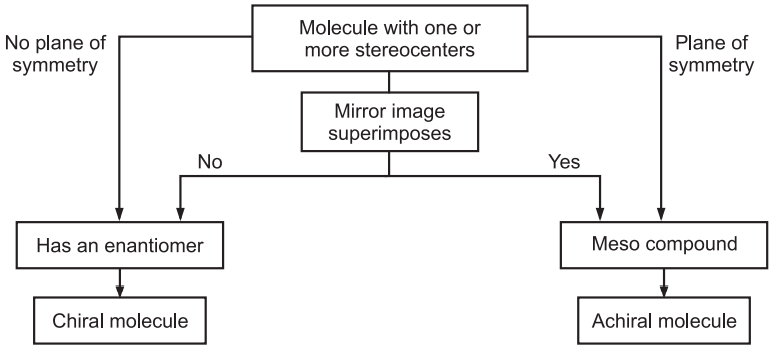
A molecule with zero stereocenters is always achiral. The molecule with an odd number of stereoisomers (1, 3, 5, etc.) will always be chiral. A molecule with an even number of stereocenters may be chiral or achiral due to meso compounds. Beside this planes of symmetry and inversion centers is the parameter to determine the chirality of a molecule. Planes of symmetry are usually easier to identify than inversion centers.
The plane that cuts the molecule in half to get the same things on both sides is known as the plane of symmetry. It can be either perpendicular to the plane or within the plane. A molecule having a plane of symmetry in any conformation is usually achiral.
(iii) Inversion center:
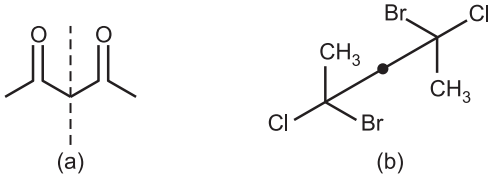
The molecule (a) has a plane of symmetry through the central carbon. This is a mirror plane where one half of the molecule is a perfect reflection of the other half of the molecule. This molecule is achiral.
The molecule (b) has a center of symmetry or an inversion center. An inversion center is a point in the molecule (may or may not be an atom) through which all other atoms can be converted through 180° into another identical part. The molecule is achiral because of the inversion center.
Make sure you also check our other amazing Article on : Stereoisomerism