Eutectic Mixtures: A two-component system containing a solid and liquid in which the two components are completely miscible in the liquid states and are completely immiscible in the solid-state. This is because the solid phase consists of pure components. This mixture is known as the eutectic mixture. The temperature at which such a system exists in the liquid phase is known as eutectic temperature. Above this temperature, the components are liquid and below this temperature they are solids. Physically eutectic systems are solid dispersions. Some examples of this type are thymol – salol, thymol – camphor, menthol – camphor, etc.
In Fig.1, the melting temperature of two substances A and B are plotted against mixture compositions. The curves separating the regions of A + Liquid and B + Liquid from regions of liquid AB are termed liquidus curves. The horizontal line separating the fields of A + Liquid and B + Liquid from A + B all solid is termed the solidus. Upon addition of B to A or A to B, their melting points are reduced. The point, E, where the liquidus curves and solidus intersect, is termed the eutectic point. At the eutectic point in this two-component system, all three phases, that is Liquid, crystals of A, and crystals of B, all exist in equilibrium. The eutectic point represents a composition (eutectic mixture composition) at which any mixture of A and B has the lowest melting point. Note that the eutectic is the only point on the diagram where this is true. At the eutectic point, the maximum numbers of allowable phases are in equilibrium. When this point is reached, the temperature must remain constant until one of the phases disappears. A eutectic is an invariant point. Below eutectic temperature, no liquid phase exists.
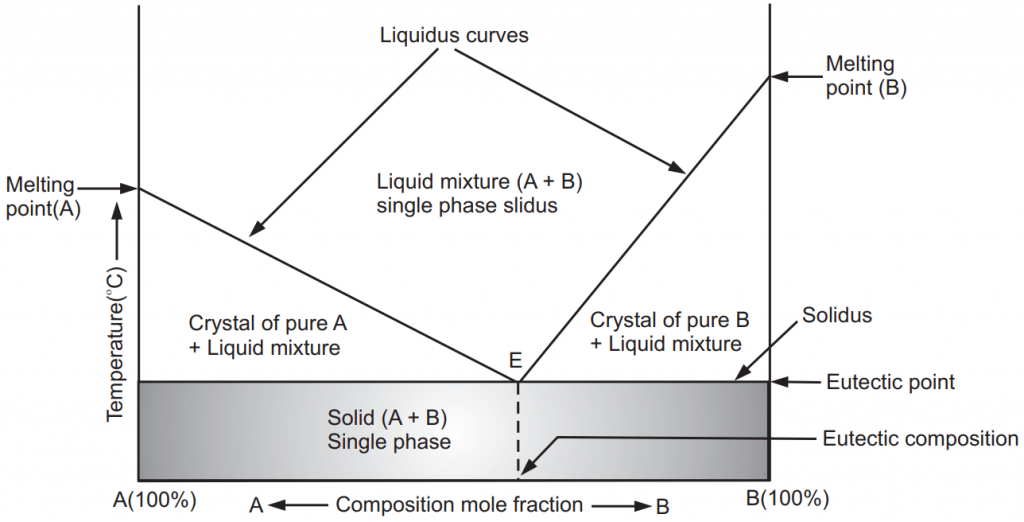
If we cool the solution of A and B which is richer in A than the eutectic mixture, then the crystal of pure A will appear. As the solution is cooled further, more and more of A get crystallize out and the solution becomes richer in B. When the eutectic point is reached, the remaining solution crystallizes out forming a microcrystalline mixture of pure A and pure B. If salol – thymol combinations are to be dispensed as a dry powder, the ambient temperature must be below its eutectic point of 13 o C. Above this temperature, it exists in liquefied form. At the eutectic point, their contribution for composition is 34 % thymol and 66 % salol.
Make sure you also check our other amazing Article on : Crystalline vs Amorphous Solids