Evaluation of Crude Drugs: Drug Evaluation is completely defined as the confirmation and complying of the drug (identity), determination of its quality, purity, and detection of the nature of adulteration. In another way, drug evaluation can be expressed as a method of estimation of active principles present in the crude drug, its morphology and microscopic analysis, its physical evaluation, the biological behavior, and determination of pharmacological evaluation.
Types of Evaluation:
- Organoleptic evaluation
- Microscopic evaluation
- Physical evaluation
- Chemical evaluation
- Biological evaluation
- Analytical evaluation
Organoleptic and Morphologic Evaluations
Table of Contents
Organoleptic character (also known as sensory evaluation) and morphological character (also known as macroscopic method) are the preliminary identification methods. These two methods (organoleptic and morphologic methods) are together known as diagnostic characters of crude drugs. These refer to the evaluation of drugs by systemic morphological characters such as leaves, barks, fruits, flowers, roots, and rhizomes, etc. (by size, shape, and special features) and further identification by sensory characters like color, odor, taste, and consistency (touch and texture). The general appearance of the crude drug indicates it is likely to comply with the standard. For example, fractured surfaces in cinchona and cascara are important characteristics. Seeds of different species of caraway, dill, leaves of senna species, the disc-shaped structure of nuxvomica, the conical shape of aconite, etc., can be distinguished. Leaves of different species of menthe can be differentiated by smell. The smell can also identify the adulterated crude drugs like clove and exhausted clove. By taste, bitter drugs like quassia, chirata, kalmegh, neem, sweet tasted drugs like glycyrrhiza, Stevia, pungent tasted drugs like ginger, capsicum, spice drugs like black pepper, nutmeg, caraway, etc. can be identified. Texture and nature of fracture also provide important information like licorice is hard and its fracture is fibrous. Fracture of ipecac is brittle, aconite and nuxvomica are horny, etc.
Microscopic Evaluation
This evaluation is also known as anatomical evaluation or histological evaluation of crude drugs. This method can be used to identify the organized drug in powdered form by their histological characters or anatomical cell or tissue arrangements. Various reagents (like chloral hydrate, conc. HCl, glycerin) and stains (phloroglucinol for identification of lignified cells and tissues like xylem, phloem, etc., Iodine for detection of starch grains, etc.) can be used to differentiate cellular structure. This evaluation also covers the study of the constituents by application of the chemical method to small quantities of the powdered drug. This is known as Chemomicroscopy. The elements such as stomata, trichomes, vessels, fibers, stone cells, starch grains, medullary rays, oil cells, calcium oxalate crystals are present in powdered conditions and are used in the microscopic identification of crude drugs. For example, leaves contain epidermis cells included trichome, stomata, calcium oxalate crystals (if present); bark contains phloem elements (cinchona), stone cells (Kurchi) or sometimes both (cascara); trichome (nuxvomica); fruits contain oil cells, endosperm, pericarp, epicarp, mesocarp, etc. This evaluation method is also useful in the identification of closely related drugs or species. Hence microscopy is two types viz. Qualitative and Quantitative. Qualitative microscopy is only detection and identification of cellular structures of drugs whereas Quantitative microscopy is to determine particular cellular substances like linear measurement as the diameter of starch grains, length of fibers, vessels, quantitative microscopic constant as a stomatal index, vein islet number, palisade ratio, Lycopodium spore method, etc. using camera lucida method using two types of micrometers viz.

Physical Evaluation:
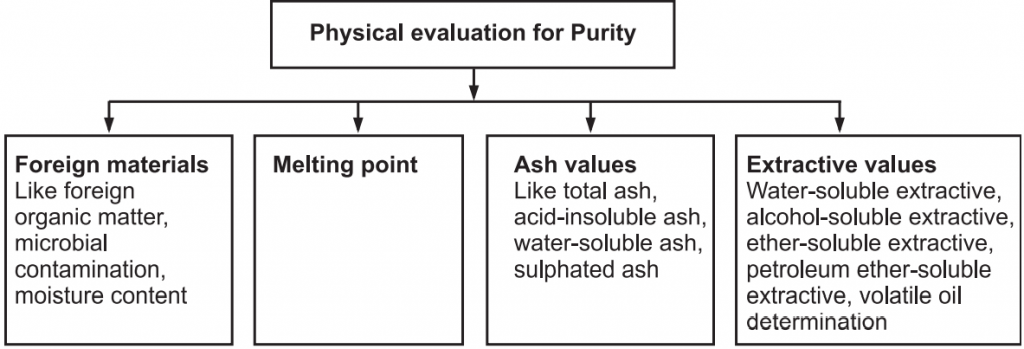
Physical standards are to be determined for drugs that are constant for crude drugs and help in drug evaluation. Physical constants such as specific gravity, refractive index, swelling factor, optical rotation, viscosity, etc., are useful in determining the identity, purity, and quality of the drugs (Figs. 1 and 2).
Chemical evaluation:
With this method active constituents in the drugs are determined. There are various methods for chemical evaluation (Fig.3).
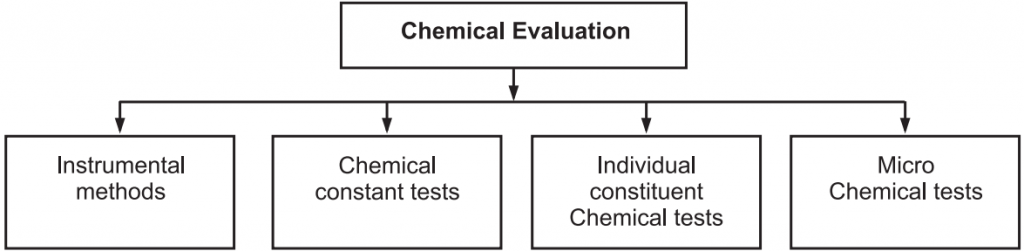
(a) Instrumental methods: Various instrumental methods like colorimetry, fluorimetry, spectrophotometry, etc. are used for the evaluation.
Colorimetric Method: It is a method of determining the concentration of a chemical element or chemical compound in a solution with the aid of a color reagent. It applies to both organic and inorganic compounds and may be used with or without an enzymatic stage. The method is widely used in medical laboratories and for industrial purposes, e.g., the analysis of water samples in connection with industrial water treatment.
Photometric Method: It is the set of methods of quantitative chemical analysis based on the relationship between the concentration of a substance in a solution or gas and the absorption of radiation. In this method, the intensities of the monochromatic components of transmitted radiation are scanned.
Fluorimetric Method: It is an analytical technique for identifying and characterizing minute amounts of a substance by excitation of the substance with a beam of ultraviolet light and detection and measurement of the characteristic wavelength of fluorescent light emitted.
Gravimetric Method: It is the quantitative determination of a substance by the precipitation method of gravimetric analysis involving isolation of an ion in solution by a precipitation reaction, filtering, washing the precipitate, conversion of precipitate to a product of known composition, and finally weighing the precipitate and determining its mass by difference. There are four fundamental types of gravimetric analysis: physical gravimetry, thermogravimetry, precipitative gravimetric analysis, and electrodeposition.
Volumetric Method: It is a quantitative analysis of liquids or solutions by comparing the volumes that react with known volumes of standard reagents, usually by titration. A reagent is prepared as a standard solution, acts as a titrator. A known concentration and volume of titrant react with a solution of analyte or titrated to determine concentration.
(b) Chemical Constant Tests: Various tests like acid value, iodine value, Saponification value, etc. are used for the evaluation of fixed oils and fats.
Saponification value: It is the number of milligrams of potassium hydroxide required to saponify 1g of fat under specific conditions. It is a measure of the average molecular weight of all the fatty acids present.
Acid value: It is the number of milligrams of potassium hydroxide (KOH) necessary to neutralize the fatty acids in 1 gram of sample.
Iodine value: It is the mass of iodine in grams that is consumed by 100 grams of a chemical substance. Iodine numbers are often used to determine the amount of unsaturation in fatty acids. The higher the iodine number, the more C=C bonds are present in the fat.
(c) Individual Constituent Chemical Tests: Various chemical tests are carried out for the identification of individual components. For each plant drug, there are some common tests for the identification of a group of plant secondary metabolites as well as specific tests for the identification of individual constituents. Molish test for detection of sugars, Lieberman Burchard test for detection of steroids, Borntrager test for detection of anthraquinones, ferric chloride test for tannins, Keller- Kiliani test for deoxy sugars, ninhydrin test for the detection of amino acids and proteins, etc.
(d) Microchemical Tests: These tests are carried out on the slide. Example: Eugenol in clove oil is precipitated as potassium alginate when potassium hydroxide is added on a slide containing clove oil. Under the microscope, clove oil shows a needle-shaped crystal of potassium alginate.

Biological evaluation:
This method is carried out when the drugs are not evaluated by any other methods, or the chemical nature of the drug is unknown or chemical methods are not available or drugs that have different chemical compositions but the same biological activity. Generally in this method, the response produced by the test drug on a living system is compared with that of the standard preparation.
This method is used for:
- Measurement of the pharmacological activity of new or chemically undefined substances.
- Investigation of the function of endogenous mediators.
- Determination of the side effect profile, including the degree of drug toxicity.
- Measurement of the concentration of known substances.
- Assessing the number of pollutants being released by a particular source such as wastewater.
Analytical Evaluation:
In this method various chromatographic, as well as spectroscopic methods, are used for the sample.
Thin Layer Chromatography (TLC): It is a chromatographic technique used to separate mixtures with the principle of adsorption. Thin-layer chromatography is performed on a sheet of glass, plastic, or aluminum foil which is coated with a thin layer of adsorbent material, usually silica gel, aluminum oxide, or cellulose known as stationary phase. The sample has been applied on the plate and the mobile phase is drawn up the plate via capillary action and based on their affinity towards the stationary phase the separation is achieved.
Advantages:
- This method is easy and simple.
- Always available to be used.
- Any type of sample can be used.
- Neutral, basic, acidic or purely aqueous eluents can be employed.
High-Performance Thin Layer Chromatography (HPTLC): HPTLC system is a versatile modern analytical technique concerning its excellent automation, optimization, multi-dimensional applications. The process is based on a fundamental principle, theory of separation, experimental details, handling understanding, and implementation. Phytochemical analysis, fingerprint analysis, authentication, biomedical analysis, drug quantification, analytical analysis, quantitative analysis, and validation are carried out by this method. The application of HPTLC in a combinatorial approach provided a new dimension to its high potential. HPTLC-MS coupling, laser desorption-HPTLC chromatography coupled with chemical ionization mass spectrometry, etc. that is used in identity, purity, quality, and stability of raw materials, extracts, and finished herbal products.
Advantages:
- Simultaneous processing of sample and standard, better analytical precision and accuracy, less need of analytical standard.
- Several analysts work simultaneously.
- Lower analysis time and less cost per analysis.
- Simple sample preparation.
- No prior treatment for solvents like filtration and degassing.
- Low mobile phase consumption per sample.
- Fresh stationery and mobile phases for each analysis but no contamination.
- Visual detection possible-open system.
High-Performance Liquid Chromatography (HPLC): It is a highly improved form of column chromatography. By this method, solvent allowed passing through the stationary column with high force under high pressure of up to 400 atmospheres. The column is made up of silica particle tightly packed material which gives a much greater surface area for interactions between the stationary phase and the molecules flowing past it. It is a chromatographic technique used to separate a mixture of compounds in analytical chemistry and biochemistry to identify, quantify, and purifying the individual components of the mixture. Depends on the relative polarity of the solvent, HPLC variants are two types (a) Normal phase HPLC and (b) Reversed-phase HPLC
Gas Chromatography (GC): It is used for the separation and analysis of vaporized compounds that are non-decomposable. The purity of a substance and separating the different components of a mixture (the relative amounts of such components can also be determined) is carried out by GC. It helps in identifying a compound. Preparative GC is used to prepare pure compounds from a mixture. The mobile phase (or “moving phase”) is a carrier gas, usually an inlet gas such as helium or an inert gas such as nitrogen. The stationary phase is a liquid or polymer on an inert solid support, called a column. It is also known as vapor[1]phase chromatography or gas-liquid partition chromatography (GLPC).

Spectroscopic Methods:
UV-visible Spectrophotometer: This method refers to absorption spectroscopy or reflectance spectroscopy in the UV-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared (NIR)) ranges. 190 nm to 380 nm in the UV region and 380 nm to 900 nm is known as the visible region. The absorption in the visible range directly affects the color of the chemicals. In this region, molecules undergo electronic transitions. It is applicable for the determination of transition metal ions, highly conjugated organic compounds, and biological macromolecules. Examples: Morphine is determined at 286 nm, Anthraquinones are determined at 505 nm,
Infra-Red Spectroscopy (IR): It is the spectroscopy that deals with the infrared region of the electromagnetic spectrum i.e., light with a longer wavelength and lower frequency than visible light. It is used to identify and study chemicals. Fourier transform infrared (FTIR) spectrometer is used in this method. The three regions of IR are the near, mid, and far regions, named for their relation to the visible spectrum. The near IR (14000– 4000 cm−1) range molecules excite harmonic vibrations whereas mid-infrared (4000–400 cm−1) is used for the fundamental vibrations associated with the rotational vibrational structure. Thereafter far-infrared (400–10 cm-1) is considered as the microwave region, used for rotational spectroscopy. It is used for identification of functional group and structure elucidation, identification of substances, detection of impurities, etc.
Nuclear Magnetic Resonance Spectroscopy (NMR): This technique deals with the magnetic properties of certain atomic nuclei to determine the physical and chemical properties of atoms or the molecules in which they are contained. It provides information about the structure, dynamics, reaction, and chemical nature of molecules. Most frequently, NMR spectroscopy is used by chemists and biochemists to investigate the properties of organic molecules, though it applies to any kind of sample that contains nuclei possessing spin. Proton and carbon NMR nuclei (1H or 13C) absorb electromagnetic radiation at a frequency characteristic of the isotope. The frequency and resonant energy of absorption and the intensity of the signal are proportional to the strength of the magnetic field.
Mass Spectroscopy: It is an analytical technique that measures the mass to charge ratio of charged particles. It is used for the determination of particle masses, the elemental composition of the molecule, and the elucidation of the chemical structure of molecules like peptides and other chemical compounds. MS works by ionizing chemical compounds to generate charged molecules or molecule fragments and measuring their mass-to-charge ratios. The technique has both qualitative and quantitative uses for the identification of unknown compounds, the isotopic composition of elements in a molecule determination, and the determination of the structure of a compound by their fragmentations.
X-ray Crystallography: It is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. The mean positions of the atoms in the crystal, as well as their chemical bonds and disorder, are determined. This method is useful for protein structure analysis.
LC-MS: It is a chemistry technique that combines the physical separation capabilities of liquid chromatography (or HPLC) with the mass analysis capabilities of mass spectrometry. LC-MS is used for the determination of high sensitivity and selectivity of the molecule. Generally, its application is oriented towards the general detection and potential identification of chemicals in the presence of other chemicals (in a complex mixture). For fast and mass purification of natural products extracts, new molecular entities important to food and pharmaceutical industries, preparative LC-MS system is used. LC-MS is very commonly used n pharmacokinetic studies of pharmaceuticals, Proteomics, and is thus the most frequently used technique in the field of bioanalysis.
Make sure you also check our other amazing Article on : Classification of Drugs
