Freeze Dryer: Freeze-drying or lyophilization is a drying process used to convert solutions of labile materials into solids of sufficient stability for distribution and storage.
Principle
Table of Contents
The main principle involved in lyophilization is a phenomenon called sublimation. The water passes directly from the solid-state (ice) to the vapor state without passing through the liquid state. Water is removed from the frozen state material and then subjected to high vacuum to heat (by conduction or radiation or by both) so that the sublime frozen liquid leaves only solids or the dry components of the original liquid. Drying is achieved by subjecting the material to temperature and pressures below the triple point.
Construction of Freeze Dryer
The freeze dryer consists of a drying chamber in which shelves are used to place the material. Heat supply is in the form of a radiation source by using heating coils. The condenser is also attached. The condenser consists of a large surface cooled by solid carbon dioxide slurred with acetone or ethanol, The condenser surface should be cleaned properly. The purpose of the condenser is to attract the vapors being sublimed off of the product. Because the condenser is maintained at a lower energy level relative to the product ice, the vapors condense and turn back into a solid form (ice) in the condenser. In shelf freeze dryers, the condenser can be located inside the product chamber (internal condenser) or in a separate chamber (external condenser) connected to the product chamber by a vapor port. The distance between subliming surface and condenser should be less than the mean path of molecules. Because this increases the rate of drying. The vacuum pump is also connected which causes evaporative cooling. The vacuum system consists of a separate vacuum pump connected to an airtight condenser and an attached product chamber.
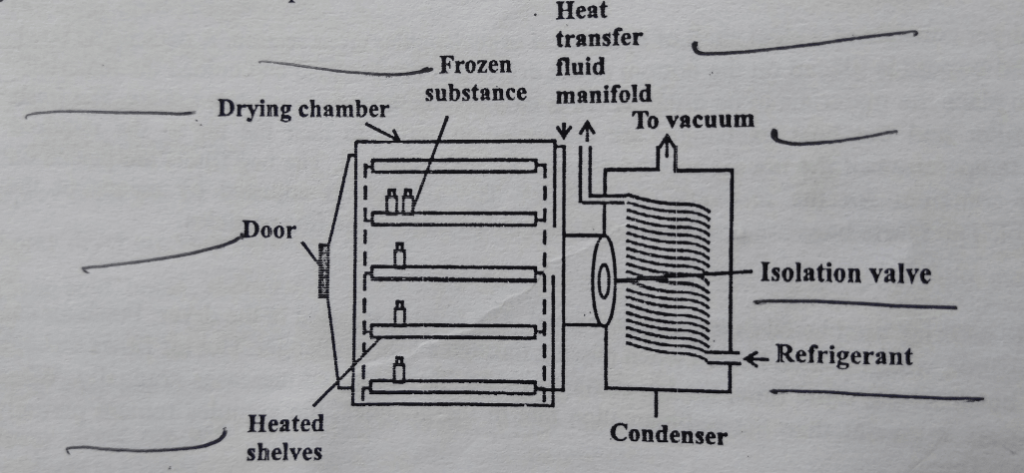
Working of Freeze Dryer
The following step is involved in the working of the freeze dryer. Preparation and pretreatment ↓ Pre freezing for solidifying water ↓ Primary Drying ↓ Secondary Drying ↓ Packing
1. Pretreatment
It includes any method of treatment of the product before freezing. This may include concentrating the product, the revision of the formulation (ie, the addition of components to increase stability and/or improve processing), decreasing a high vapor pressure solvent, or increasing the surface area. Pretreatment methods include freezing concentration, solution-phase concentration, formulation to preserve the appearance of the product, formulation to stabilize reactive products, formulation to increase the surface area, and decreasing high vapor pressure solvent.
2. Pre freezing:
The product should be frozen at a temperature low enough to solidify completely. Since freeze-drying is a change in the state from the solid phase to the gas phase, the material to be freeze-dried must first be adequately pre-frozen. The pre-freezing method and the final temperature of the frozen product can affect the ability to successfully freeze the material. The rapid cooling results in small ice crystals, useful for preserving structures that will be examined microscopically, but it produces a product that is more difficult to lyophilize. Slower cooling results in large ice crystals and a less restrictive channel in the matrix during the drying process. The products are frozen in two ways, most of the products that are lyophilized consist mainly of water. Most of the samples to be lyophilized are eutectic, which are mixtures of substances that freeze at a lower temperature than the surrounding water. It is very important in lyophilization to pre-freeze the product below the eutectic temperature before beginning the lyophilization process. The second type of frozen product is a suspension that is subjected to the formation of glass during the freezing process.
3. Primary drying:
After pre-freezing the product, conditions must be established in which the ice can be removed from the frozen product through sublimation, resulting in a dry, structurally intact product. This requires very careful control of the two parameters: Temperature and Pressure involved in the lyophilization system. The sublimation rate of a frozen product depends on the difference in vapor pressure of the product compared to the vapor pressure of the ice collector. The molecules migrate from the high-pressure à lower pressure area. As the vapor pressure is related to the temperature, the temperature of the product must be warmer than the temperature of the cold trap (ice collector). The temperature at which a product is lyophilized is balanced between the temperature that maintains the frozen integrity of the product and the temperature that maximizes the vapor pressure of the product. This balance is key to optimum drying.
Heat enters the products by one of several mechanisms:
- By direct contact between the container base and the shelf or
- By conduction across the container base and then through the frozen mass to the drying front or
- By gaseous convection between the product and residual gas molecules in the chamber or
- By radiation.
4. Secondary Drying:
After primary freeze-drying is complete, and all ice has sublimed, bound moisture is still present in the product. The product appears dry, but the residual moisture content may be as high as 7-8%. Continued drying is necessary at a warmer temperature to reduce the residual moisture content to optimum values. This process is called ‘Isothermal Desorption’. Secondary drying is usually carried out for approximately 1/3 or 1/2 of the time required for primary drying.
5. Packing:
By replacing vacuum with inert gas, bottles and vials are closed.
Advantage of Freeze Dryer
- This is suitable for drying heat sensitive products
- Freeze-dried product is porous and easy to be rehydrated and instantly dissolved.
- Drying takes place at very low temperatures, so that enzyme action is inhibited and chemical decomposition, particularly hydrolysis, is minimized.
- Denaturation of protein does not occur.
- Loss of volatile material is less.
- Sterility can be maintained.
Disadvantage of Freeze Dryer
- The process is very slow
- Expensive process.
- It is not a general method of drying, but it is limited to certain types of valuable products that can not be dried by any other means.
- The period of drying is high
- The product is prone to oxidation, due to the high porosity and large surface area. Therefore, the product must be vacuum packed or with inert gas or in a container
Pharmaceutical Applications
- It is used in the production of injection, solution, and suspension. It is also used for the production of blood plasma and its fractionated products, bacterial and viral cultures, antibiotics and plant extracts, steroids, vitamins, and enzymes.
- Food items like mushrooms, prawns, meat products can be dried by this method 3. Coffee and tea concentrate and citrus fruit juices are also dried by this method.
Make sure you also check our other amazing Article on : Vacuum Dryer
