Gastro Retentive Drug Delivery System (GRDDS) has gained immense popularity in the field of oral drug delivery recently. Gastro Retentive Drug Delivery System is a widely employed approach to retain the dosage form in the stomach for an extended period and release the drug slowly that can address many challenges associated with conventional oral delivery, including poor bioavailability. Different innovative approaches like magnetic field-assisted gastro-retention, plug type swelling system, muco-adhesion technique, a floating system with or without effervescence are being applied to fabricate GRDDS.
Gastro-retentive drug delivery is an approach to prolong gastric residence time, thereby targeting site-specific drug release in the upper gastrointestinal tract (GIT) for local or systemic effects. These drug delivery systems suffer from mainly two adversities: the short gastric retention time (GRT) and unpredictable short gastric emptying time (GET), which can result in incomplete drug release from the dosage form in the absorption zone (stomach or upper part of small intestine) leading to diminished efficacy of administered dose. To formulate a site-specific orally administered controlled release dosage form, it is desirable to achieve a prolonged gastric residence time by the drug delivery. Prolonged gastric retention improves bioavailability, increases the duration of drug release, reduces drug waste, and improves the drugs that are less soluble in a high pH environment. Also, prolonged gastric retention time (GRT) in the stomach could be advantageous for local action in the upper part of the small intestine e.g. treatment of peptic ulcer, etc.
Advantages of Gastro Retentive Drug Delivery System
Table of Contents
- Enhanced bio-availability.
- Reduced frequency of dosing.
- Targeted therapy for local ailments in the upper GIT.
- Patient compliance.
- Improved therapeutic efficacy.
Gastro retentive drug delivery system (GRDDS) greatly improves pharmacotherapy of the stomach through local drug release leading to high drug concentrations at gastric mucosa (eradicating helicobacter pylori from the submucosal tissue of the stomach), making it possible to treat stomach and duodenal ulcers, gastritis, and esophagitis, reduce the risk of gastric carcinoma, controlled release antacid formulations. GRDDS can be used as carriers for drugs that are absorbed from absorption windows in the stomach. For example, various antibiotics, antiviral and antifungal agents, etc. (sulphonamides, quinolones, penicillins, cephalosporins, aminoglycosides, tetracyclines, etc.) are taken up only from very specific sites of the GI mucosa.
Disadvantages of Gastro Retentive Drug Delivery System
There are certain situations where gastric retention is not desirable. Aspirin and nonsteroidal anti-inflammatory drugs are known to cause gastric lesions and slow release of such drugs in the stomach is unwanted. Thus, drugs that may irritate the stomach lining or are unstable in their acidic environment should not be formulated in gastro-retentive systems. Furthermore, other drugs such as; isosorbide dinitrate that is absorbed equally well throughout the GIT will not be suitable for incorporation into a gastric retention system.
Also, GRDD’s have some limitations such as:
- Requirement of high levels of fluids in the stomach for the delivery system to float and work efficiently.
- Requires the presence of food to delay gastric emptying.
- Drugs, which undergo significant first-pass metabolism, may not be desirable candidates for floating drug delivery systems since the slow gastric emptying.
- May lead to altering systemic bioavailability.
- Drugs having solubility or stability problems in the highly acidic gastric environment or which are irritants to gastric mucosa cannot be formulated as GRDDS.
Factors Controlling Gastric Retention of Dosage Forms
(a) Density of Dosage Form:
- Dosage forms having a density lower than that of gastric fluid experience floating behavior and hence gastric retention.
- A density of <1.0 gm/ml is required to exhibit floating property.
- However, the floating tendency of the dosage form usually decreases as a function of time, as the dosage form gets immersed into the fluid, as a result of the development of hydrodynamic equilibrium.
(b) Shape and Size of the Dosage Form:
- The mean gastric residence times of non-floating dosage forms are highly variable and greatly dependent on their size, which may be large, medium, and small units.
- In most cases, the larger the dosage form the greater will be the gastric retention time (GRT) due to the larger size of the dosage form would not allow this to quickly pass through the pyloric antrum into the intestine.
- Ring-shaped and tetrahedron-shaped devices have a better gastric residence time as compared with other shapes.
(c) Food Intake and Nature of Food:
- Food intake, the nature of the food, caloric content, and frequency of feeding have a profound effect on the gastric retention of dosage forms.
- The presence or absence of food in the stomach influences the GRT of the dosage form.
- Usually, the presence of food increases the GRT of the dosage form and increases drug absorption by allowing it to stay at the absorption site for a longer time.
(d) Effect of Gender, Posture, and Age:
- Generally, females have slower gastric emptying rates than males.
- The effect of posture does not have any significant difference in the mean gastric retention time (GRT) for individuals in upright, ambulatory, and supine states.
- In the case of elderly persons, gastric emptying is slowed down.
Approaches for Gastro Retentive Drug Delivery System
- Floating drug delivery systems.
- Mucoadhesive systems.
- Swellable systems.
- High-density systems.
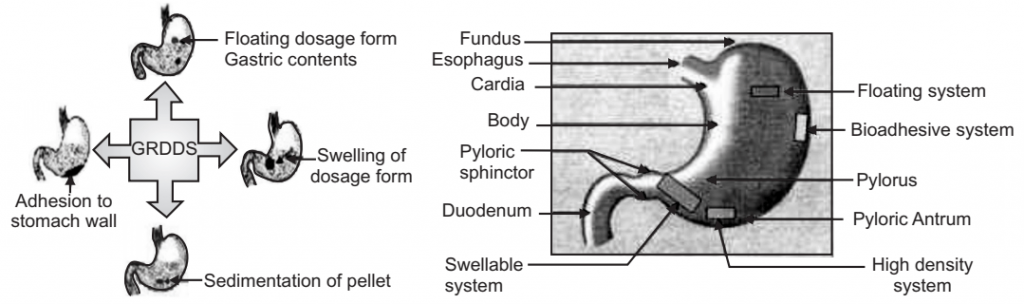
These are explained in the articles given below.
Floating Drug Delivery System
Floating drug delivery systems (FDDS) have a bulk density lower than gastric fluids and thus remain buoyant in the stomach for a prolonged period, without affecting the gastric emptying rate. While the system floats on gastric contents, the drug is released slowly at a desired rate from the system. After the release of the drug, the residual system is emptied from the stomach. This increases gastric retention time and better control of fluctuations in plasma drug concentrations. Floating systems can be classified into two distinct categories, (i) Non-effervescent and (ii) Effervescent systems.
Effervescent:
- Gas generating systems.
- Volatile liquid-containing systems.
- Inflatable gastrointestinal delivery systems.
- Intragastric osmotically controlled drug delivery system.
Non-Effervescent:
- Colloidal gel barrier systems.
- Alginate beads.
- Hollow microspheres.
- Microporous compartment system.
Effervescent
(a) Gas Generating Systems:
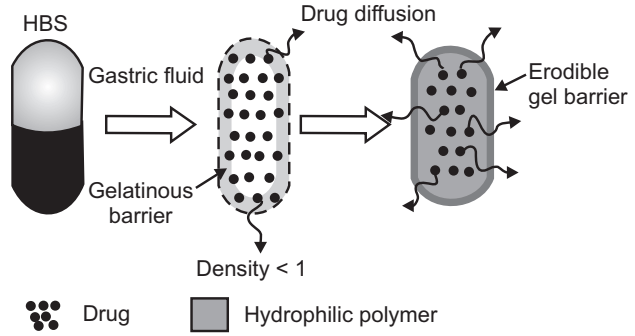
Intra Gastric Single Layer Floating Tablets or Hydrodynamically Balanced System (HBS): These are as shown in Fig.2 and formulated by intimately mixing the CO2 generating agents and the drug within the matrix. These have a bulk density lower than gastric fluids and therefore remain floating in the stomach unflattering the gastric emptying rate for a prolonged period. The drug is slowly released at a desired rate from the floating system and after the complete release, the residual system is expelled from the stomach. This leads to an increase in the gastric retention time and better control over fluctuations in plasma drug concentration.
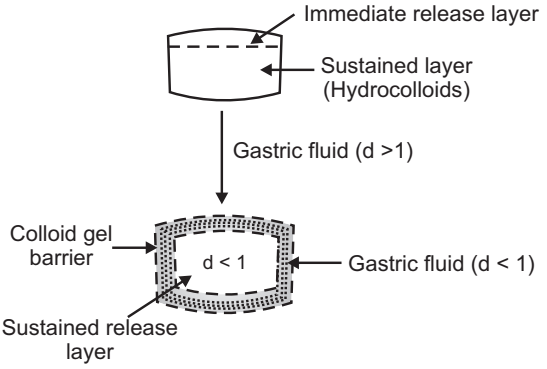
Intra Gastric Bilayer Floating Tablets:
These are also compressed tablets as shown in Fig.3. and contain two layers i.e.
- Immediate-release layer
- Sustained-release layer
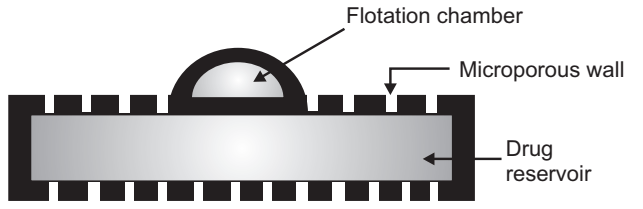
(b) Volatile Liquid/ Vacuum Containing Systems:
Intragastric Floating Gastrointestinal Drug Delivery System: These systems can be made to float in the stomach because of floatation chamber, which may be a vacuum or filled with air or a harmless gas, while drug reservoir is encapsulated inside a microporous compartment
(c) Inflatable Gastrointestinal Delivery Systems:
In these systems, an inflatable chamber is incorporated, which contains liquid ether that evaporates at body temperature to cause the chamber to inflate in the stomach. These systems are fabricated by loading the inflatable chamber with a drug reservoir, which can be a drug, impregnated polymeric matrix, then encapsulated in a gelatin capsule. After oral administration, the capsule dissolves to release the drug reservoir together with the inflatable chamber. The inflatable chamber automatically inflates and retains the drug reservoir compartment in the stomach. The drug is continuously released from the reservoir into the gastric fluid.
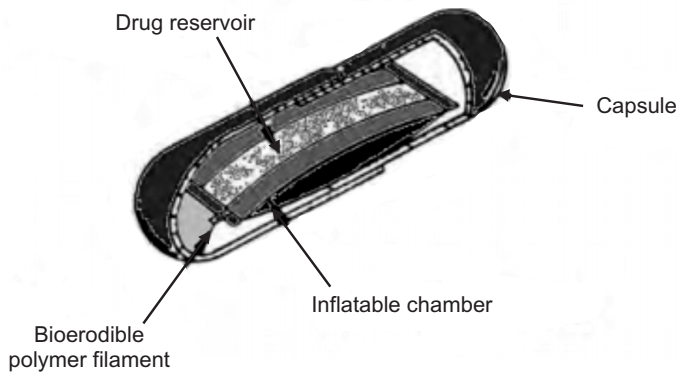
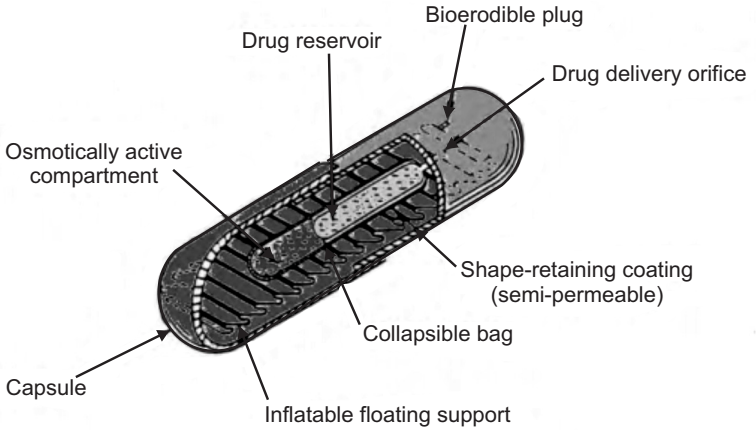
(d) Intragastric Osmotically Controlled Drug Delivery System:
It is comprised of an osmotic pressure-controlled drug delivery device and inflatable floating support in a biodegradable capsule. In the stomach, the capsule quickly disintegrates to release the intragastric osmotically controlled drug delivery device. The inflatable support inside forms a deformable hollow polymeric bag that contains a liquid that vaporizes at body temperature to inflate the bag. The osmotic pressure-controlled drug delivery device consists of two components drug reservoir compartment and an osmotically active compartment. The drug reservoir compartment is enclosed by a pressure responsive collapsible bag, which is impermeable to vapor and liquid and has a drug delivery orifice. The osmotically active compartment contains an osmotically active salt and is enclosed within a semi-permeable housing. In the stomach, the water in the gastrointestinal fluid is continuously absorbed through the semi-permeable membrane into the osmotically active compartment to dissolve the osmotically active salt. Osmotic pressure is thus created which acts on the collapsible bag which forces the drug reservoir compartment to reduce its volume and which in turn activates the drug release from the drug solution compartment through the delivery orifice. The floating support is also made to contain a bio-erodible plug that erodes after a predetermined time to deflate the support. The deflated drug delivery system is then emptied from the stomach.
Non-Effervescent
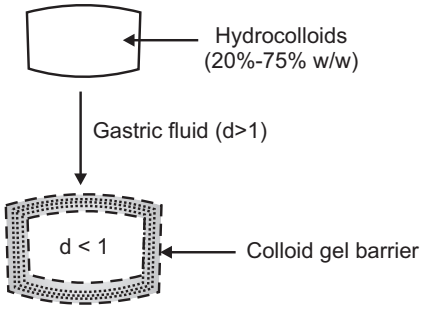
(a) Colloidal Gel Barrier Systems:
- Such systems contain drugs with gel-forming hydrocolloids meant to remain buoyant on stomach contents.
- These systems incorporate a high level of one or more gel-forming highly swellable cellulose-type hydrocolloids. For e.g. HPMC, NaCMC.
- On coming in contact with gastric fluids forms a viscous core.
- Incorporates H2O and entraps air.
- The density of the system falls below 1 gm/cm3. Then it starts floating.
(b) Microporous Membrane System:
Based on the encapsulation of drug reservoir inside a Microporous compartment,
- The peripheral walls of the drug reservoir compartment are completely sealed to prevent any direct contact of the gastric mucosal surface with the undissolved drug.
- In the stomach, the floatation chamber containing entrapped air causes the delivery system to float over the gastric contents.
- Gastric fluid enters through the apertures, dissolves the drug, and carries the dissolved drug for absorption.
(c) Alginate Beads:
- Spherical beads of approximately 2.5 mm in diameter can be prepared by dropping a sodium alginate solution into aqueous solutions of calcium chloride, causing precipitation of calcium alginate.
- Sodium alginate Calcium chloride, Calcium alginate NaCl.
- The beads are then separated and frozen in liquid nitrogen, and freeze-dried at −40°C for 24 hours, leading to the formation of a porous system.
- Maintain a floating force of over 12 hours.
(d) Hollow Microspheres:
- Micro balloons/ hollow microspheres loaded with drugs are prepared by a simple solvent evaporation method.
- Commonly used polymers to develop these systems are polycarbonate, cellulose acetate, calcium alginate, Eudragit S, agar and pectin, etc.
- These systems can float on acidic dissolution media containing surfactant for about 12 hours invitro.
Bioadhesive or Mucoadhesive Drug Delivery Systems
Bioadhesive drug delivery systems are used as a delivery device within humans to enhance drug absorption in a site-specific manner. In this approach, bio-adhesive polymers are used and they can adhere to the epithelial surface in the stomach.
Thus, they improve the prolongation of gastric retention. The basis of adhesion is that a dosage form can stick to the mucosal surface by different mechanisms. These mechanisms are:
- The wetting theory is based on the ability of bioadhesive polymers to spread and develop intimate contact with the mucous layers.
- The diffusion theory proposes physical entanglement of mucin strands the flexible polymer chains, or interpenetration of mucin strands into the porous structure of the polymer substrate.
- The absorption theory, suggests that bio-adhesion is due to secondary forces such as; van der Waal forces and hydrogen bonding.
- The electron theory proposes attractive electrostatic forces between the glycoprotein mucin network and the bio-adhesive material.
Materials commonly used for bioadhesion are polyacrylic acid, chitosan, cholestyramine, sodium alginate, hydroxypropyl methylcellulose (HPMC), sucralfate, tragacanth, dextrin, polyethylene glycol (PEG) and polylactic acids, etc. Even though some of these polymers are effective at producing bioadhesives, it is very difficult to maintain them effectively because of the rapid turnover of mucus in the gastrointestinal tract (GIT).
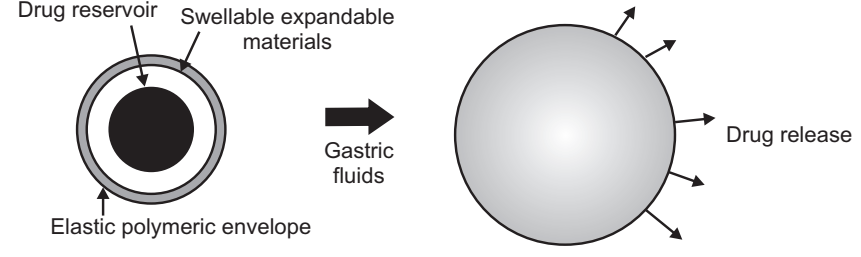
Expandable, Unfoldable, and Swellable Systems
A dosage form in the stomach will withstand gastric transit if it is bigger than a pyloric sphincter. However, the dosage form must be small enough to be swallowed, and must not cause gastric obstruction either singly or by accumulation. Thus, their configurations are required to develop an expandable system to prolong gastric retention time (GRT).
- A small configuration for oral intake.
- An expanded gastro-retentive form.
- A final small form enabling evacuation following drug release from the device.
Thus, gastro-retentivity is improved by the combination of substantial dimension with high rigidity of dosage form to withstand peristalsis and mechanical contractility of the stomach. Unfoldable and swellable systems have been investigated and recently tried to develop an effective gastro-retentive drug delivery.
Unfoldable systems are made of biodegradable polymers. They are available in different geometric forms like; tetrahedron, ring, or planner membrane (4 – label disc or 4 – limbed cross form) of bioerodible polymer compressed within a capsule that extends in the stomach. Swellable systems are also retained in the gastrointestinal tract (GIT) due to their mechanical properties. The swelling is usually resulting from osmotic absorption of water and the dosage form is small enough to be swallowed by the gastric fluid. Expandable systems have some drawbacks like problematical storage of much easily hydrolyzable, biodegradable polymers, relatively short-lived mechanical shape memory for the unfolding system, most difficult to industrialize, and not cost-effective. Again, permanent retention of rigid, large single-unit expandable drug delivery dosage forms may cause brief obstruction, intestinal adhesion, and gastropathy.
Applications of Gastroadhesive Systems
Enhanced Bioavailability
- The bioavailability of riboflavin GRDF is significantly enhanced in comparison to the administration of non-GRDF polymeric formulations.
- There are several different processes, related to the absorption and transit of the drug in the gastrointestinal tract, that act concomitantly to influence the magnitude of drug absorption.
Sustained Drug Delivery/Reduced Frequency of Dosing
- For drugs with a relatively short biological half-life, sustained and slow input from GRDF may result in improved pharmacokinetics and reduced dosing frequency.
- This feature is associated with improved patient compliance and thereby improves therapy.
Targeted Therapy for Local Ailments in the Upper GIT
- The prolonged and sustained administration of the drug from GRDF to the stomach may be advantageous for local therapy in the stomach and small intestine.
- By this mode of administration, therapeutic drug concentrations may be attained locally while systemic concentrations, following drug absorption and distribution, are minimal.
Reduced Fluctuations of Drug Concentration
- Continuous input of the drug following GRDF administration produces blood drug concentrations within a narrower range compared to the immediate release dosage forms.
- Thus, fluctuations in drug effects are minimized and concentration-dependent adverse effects that are associated with peak concentrations can be prevented.
- This feature is of special importance for drugs with a narrow therapeutic index.
Make sure you also check our other amazing Article on : Implantable Drug Delivery System
