Hair dye has been used since ancient Egyptian times when Rameses II reinforced red hair colour using henna. In ancient Greece, the hair was bleached with a rinse of potassium solution and rubbed with a type of ointment made of yellow flower petals and pollen. Some of the most well known are henna (Lawsonia inermis), indigo, Cassia obovata, senna, turmeric and amla. Others include katam, black walnut hulls, red ochre and leeks. The development of synthetic dyes for hair is traced to the 1860s discovery of the reactivity of para-phenylenediamine (PPD) with air. Since Second World War, great progress in discoveries and applications of new synthetic dyes has taken place.
Dye Composition
The preparation (dye precursors) is in the leuco (colourless) form. Oxidizing agents are usually hydrogen peroxide, and the alkaline environment is usually provided by ammonia. The combination of hydrogen peroxide and ammonia causes the natural hair to be lightened, providing a “blank canvas” for the dye. The developer is a mixture of hair colours does exactly what the name implies. It provides the chemical reaction that allows the hair colour molecules to penetrate and be deposited into the shaft of the hair by causing the chemical colour to “develop”. Depending on the strength of the peroxide of the developer, the developer also may lighten the base colour of the hair to create a lighter shade of colour. The formulation and balance between hair colour and developer are designed to use equal parts of each separate mixture of components. Therefore two ounces of hair colour (which contains ammonia and oxidative tints (usually aniline derivatives) is meant to be combined with two ounces of developer (hydrogen peroxide). This results in the oxidation of the aniline derivatives which creates the colour to be deposited into the hair. (The aniline derivatives combine with peroxide to form larger colour molecules.) When you use less developer than is called for, you will not get as much reaction from the chemicals combined, which can result in duller colour results and less penetration of the colour. Using more, however, can dilute the colours and result in paler results than intended. Peroxide developer volumes indicate the developers oxidizing potential. Peroxide breaks down melanin in the hair as it oxidizes. The higher the volume, the more lift in colour it provides. The low volume provides no lift and simply opens the cuticle so that the dye can be deposited in the cortex.
Classification of Hair Dyes
The hair dyeing systems can be classified as follows:
(i) According to mechanism:
- oxidative (containing hydrogen peroxide)
- non-oxidative.
(ii) According to the colour durability after the application on hair strands:
- temporary
- demi-permanent (sometimes called deposit only)
- semi-permanent
- permanent.
Many studies have established the diffusion path of the dye molecule to the inner hair fibre. It involves the permeation of the molecules into intercuticular regions, passing through non-keratinized regions of the endocuticle and the intracellular cement. In later stages, it migrates to keratinized regions and, eventually, reaches the macro fibrils, before being incorporated into the matrix. The temporary and semi-permanent non-oxidative dyes are based on colourful molecules, named dye deposition because the dye molecule only interacts with the hair cuticles. When there is a small penetration of the molecules into the hair cortex, they are named semi-permanent products and can be resistant to up to six washes. The demi-permanent and permanent oxidative are based on precursors, named oxidation dyes, whose colour characteristics are developed using the interaction with an oxidizing agent, and present longer-lasting colour.
1. Permanent Hair Dyes:
Permanent oxidative hair dyes are commonly used because this category provides greater efficacy of permanent dyeing, resistance to shampoo washes and other external factors, such as drying, friction, light, and others. This category represents about 80% of the sold hair dyes and gets any shade, covering up to 100% of white hair strands. Also, it is possible to have dark and light natural hair colour due to the combination of the oxidizing agents with the ammonia hydroxide. The principal difference between the semi-permanent hair dye in comparison with a permanent one is the alkalizing agent used because, in the first, monoethanolamine with low colour lightening power is used. Colour formation happens upon a mixture and involves complex reactions between precursors in the presence of an oxidizing agent. The precursors can be classified into two categories: oxidation basis or primary intermediaries, and the couplers or reaction modifiers. The reaction occurs in an alkaline medium that promotes the opening of the cuticles that allows the penetration of the dyes molecules into the cortex. The oxidizing agent permits the beginning of the reaction that occurs in the cortex and results in a colourful complex with high molar mass, which avoids the exit of molecules formed in the hair. Part of the reaction also happens on the cuticles and the molecules are removed in the first wash. The ammonia hydroxide and ethanolamines are the most alkalizing agents used. A mixture of surfactants and solvents is used to disperse the dye molecules and ensure hair wetting. A small amount of reducing agent is added to prevent auto-oxidation of the dyes during storage of the finished product, which may be formulated as an emulsion, gel, solution and powder. The reactions involved in the formation of permanent dyes are redox types and require four major components: the aromatic amine with substitutions at positions ortho or para (hydroxy or amino) as the coupling bases; the reaction modifiers; an alkalizing compound; and an oxidizing agent.
Permanent hair colour generally contains ammonia and must be mixed with a developer or oxidizing agent to permanently change hair colour. Ammonia is used to open the cuticle layer so that the developer and colourants together penetrate the cortex. The developer (oxidizing agent) comes in various volumes. The higher the developer volume, the higher the lift will be of a person’s natural hair pigment. Someone with dark hair wishing to achieve two or three shades lighter may need a higher developer, whereas someone with lighter hair wishing to achieve darker hair will not need a high developer. The application time with permanent hair colouring is typically 30 minutes or 45 minutes for those wishing to achieve maximum grey coverage.
2. Demi-permanent Hair Dyes:
Demi-permanent hair colour is the hair colour that contains an alkaline agent other than ammonia (e.g. ethanolamine, sodium carbonate) and, while always employed with a developer, the concentration of hydrogen peroxide in that developer may be lower than used with permanent hair colour. Since the alkaline agents employed in demi-permanent colours are less effective in removing the natural pigment of hair than ammonia, these products provide no lightening of hair’s colour during dyeing. As the result, they cannot colour hair to a lighter shade than it was before dyeing and are less damaging to hair than their permanent counterpart. Demi-permanents are much more effective at covering grey hair than semi permanents but less so than permanents.
Demi-permanents have several advantages as compared with permanent colour. Because there is essentially no lifting (i.e., removal) of natural hair colour, the final colour is less uniform/homogeneous than a permanent and therefore more natural looking; they are gentler on hair and therefore safer, especially for damaged hair; and they wash out over time (typically 20 to 28 shampoos), so root regrowth is less noticeable and if a change of colour is desired, it is easier to achieve. Demi-permanent hair colours are not permanent, but the darker shades, in particular, may persist longer than indicated on the packet.
3. Semi-permanent Hair Dyes:
Semi-permanent hair colouring involves little or no developer, hydrogen peroxide or ammonia, and is thus less damaging to hair strands. The reduced amount of developer, whether peroxide or ammonia, means that hair previously damaged by applying permanent colour or permanent reshaping is less likely to be damaged during the colour application process. Semi-permanent hair colour uses compounds of low molecular weight that are found in temporary hair colour dyes. These dyes penetrate the hair shaft only partially, because of the reduced amount of developer used. For this reason, the colour will survive repeated washing, typically 4–5 shampoos or a few weeks, before undergoing significant fading or washing out entirely.
The final colour of each strand of hair will depend on its original colour and porosity. Because of hair’s colour and porosity across the head and along the length of a hair strand, there will be subtle variations in shade across the entire head. This gives a more natural-looking result than the solid, all over colour of a permanent colour. Because grey or white hairs have a different starting colour than other hair, they will not appear as the same shade as the rest of the hair when treated with semi-permanent colour. If there are only a few grey/white hairs, the effect will usually be enough for them to blend in, but as the grey spreads, there will come a point where it will not be disguised as well. In this case, the move to permanent colour can sometimes be delayed by using the semi-permanent as a base and adding highlights. Semi-permanent colour cannot lighten the hair.
Semi-permanent non-oxidative hair dyeing formulations contain basic or cationic dyes with low molar mass, which have a high affinity for hair keratin and resist from three to six washes. The hair dyeing process does not involve an oxidation reaction; the application is simple and lasts from 10 to 40 min, followed by rinsing. Several products are available in the market: lotions, shampoos, mousses and emulsions. These cosmetic forms must have the ideal viscosity so that they do not flow during the application. Dyes with low molar mass penetrate slightly in the cortex, especially because of the high pH value of the product that promotes the opening of the cuticles. Semi-permanent hair products promote major hair colour durability (resistance up to 20 washes) because they consist of a mix of semi-permanent molecules with oxidation dye precursors, applied with hydrogen peroxide (H2O2). Another option of formulation involves mixing nitro aniline dyes with basic or acid dyes which aim for a better colour result and a bigger resistance to washes, considering the high affinity of the two families of dyes. The nitro anilines are molecules comprised of neutral aromatic amine or anthraquinone derivatives and all are classified as highly polar and present mono, di, or trinuclear rings. These dyes are diffused through the hair fibre and are retained by weak Van der Waals bonds. Under similar conditions, the larger molecules with tri-aromatic rings are removed more slowly from hair than the smaller, mononuclear ones. Examples of semipermanent hair dyeing are 4-hydroxypropylamino-3-nitrophenol, N, N’-bis-(2-hydroxyethyl)- 2-nitrophenylenediamine, HC Yellow No. 2 and HC Red No. 2.
Semi-permanents may contain the suspected carcinogen p-phenylenediamine (PPD) or other related colourants. The U.S. Environmental Protection Agency reported that in rats and mice chronically exposed to PPD in their diet, the PPD appeared to depress the body-weight of the animals, with no other clinical signs of toxicity observed in several studies.
4. Temporary Colour Hair Dyes:
Temporary hair colour is available in various forms including rinses, shampoos, gels, sprays, and foams. Temporary hair colour is typically brighter and more vibrant than semi-permanent and permanent hair colour. The pigments in temporary hair colour are high molecular weight and cannot penetrate the cuticle layer. The colour particles remain adsorbed to the surface of the hair shaft and are easily removed with a single shampooing because dye presents high molecular weight and deposits on the hair surface without the capacity of penetrating the cortex. This type of dye does not have the power of whitening the hair strand and, therefore, it is indicated only to add new nuance and not to change its colour. Temporary hair colour can persist on hair that is excessively dry or damaged in a way that allows for migration of the pigment to the interior of the hair shaft. The temporary dye can be used for specific purposes such as adding colourful reflections, removing the yellowish effects of the white hair, and covering a small quantity of white hair. These dyes, that present acid characteristics usually have high molar mass. They contain anionic characteristics and are selected to allow the maximum solubility in water and the minimum penetration in the hair so it is removed in the first washing. They are presented as shampoo, gel, emulsion and solution (liquid) with two different forms of application: continuous application (progressive) or single application, with one wash at the end of the application process to remove the unabsorbed dye excess on the hair strand. Examples of these dyes are acid yellow 1, acid yellow 23, acid orange 7, acid red 33, acid red 92 and acid blue 9.
5. Alternative Colour Hair Dyes:
Alternative hair colouring products are designed to create hair colours not typically found in nature. The available colours are diverse, such as the colours green and fuchsia. Permanent alternatives in some colours are available. Some colour shades are blacklight-reactive, and thus show up under certain nightclub lighting, for instance. The chemical formulae of alternative colour dyes typically contain only tint and have no developer. This means that they will only create the bright colour of the packet if they are applied to light blond hair. People with darker hair (medium brown to black) need to use a bleaching kit before tint application. Some people with fair hair may benefit from prior bleaching as well. Gold, yellow and orange undertones in hair that has not been lightened enough can adversely affect results, especially with pinks, blues and greens. Although some alternative colours are semipermanent, such as blue and purple, it could take several months to fully wash the colour from bleached or pre-lightened hair.
Thus, among the various options of hair dyes, it is interesting to know the application features and their affinity for the hair fibres to select the best option for each hair type and to provide a satisfactory effect, as a good covering power of grey/white hair, good colour resistance to shampoo washes, and high durability of colour. The challenge is to find options that provide security in the application and allow these benefits to occur without generating very aggressive damage to the hair strands.
Chemistry of Permanent Hair Colouring
Permanent hair dyes are widely used with over 80% of the market share in the United States. In the 1980’s, some hair dye chemicals were banned from use due to their mutagenic and carcinogenic effects as evidenced by laboratory animal studies. However, hair dye ingredients such as para-phenylenediamine (PPD) and para-toluene diamine (PTD) are still being used to date. For the past 50 years, PPD has been used commonly as a primary intermediate in the formulation of permanent hair dyes and remains unchanged. Today, the European Union cosmetic directive regulation allows maximum PPD concentration of up to 6% in hair dyes. PPD is an aromatic amine (Fig. 5.4) with a chemical formula C6H8N2. Present in the form of white crystals, PPD oxidizes in the air turning from red to brown and finally black. PPD can penetrate the hair shaft and follicle and has a strong protein binding capacity, thereby making it an effective hair dye chemical. Additionally, it is also used in the fur and textile industries and as a vulcanizing agent in the rubber industry. Given its widespread use in the United States, Europe, and East Asia, the safety assessment of hair dye ingredients remains a growing concern. The possible association between permanent hair dye use and cancer risk has been examined in several cohort studies.
According to the Scientific Committee on Consumer Products (SCCP), PPD is a potent contact allergen. Skin contact during the hair dyeing or tattooing process is the main route of human exposure to PPD. Occupational exposure is seen among industrial workers and regular hairdressers handling this chemical. Due to the popularity of hair dye use and its ability in penetrating human skin, concerns on the hair dye toxicity, mutagenicity and/or carcinogenic potential on the human body need to be adequately considered. The PPD is especially oxidized in a reactive intermediate, the quinoamine that, in the presence of the reaction modifier, will generate a colourful polymer.
Permanent hair colouring requires three components: (1) 1, 4-diaminobenzene (historically) or 2,5-diamine toluene (currently), (2) a coupling agent, and (3) an oxidant. The process is typically accomplished under basic conditions. The mechanism of oxidation dyes involves three steps: (1) Oxidation of 1, 4-diaminobenzene derivative to the quinone state. (2) Reaction of this diimine with a coupler compound, and (3) Oxidation of the resulting compound to give the final dye.
The combination of hydrogen peroxide and ammonia causes the natural hair to be lightened, providing a “blank canvas” for the dye. Ammonia opens the hair shaft pores so that the dye can diffuse inside the fibre. The dye intermediates and coupler compounds can undergo oxidation and coupling reactions (shown in the scheme) to form high molecular weight products, which are trapped in the hair matrix and cannot be readily removed through washing.
Various combinations of primary intermediates and coupler compounds provide a spectrum of shades of hair colours. The primary intermediates are aromatic para compounds, such as 1,4-diaminobenzene or 4-aminophenol. The coupler compounds (couplers) are meta-substituted derivatives of aniline. They come in three major classes based on the colour that they produce when they react with the primary intermediate.
- Blue couplers include 1,3-diaminobenzene and its derivatives.
- Red couplers include phenols and naphthols, such as 3-aminophenol, 5-amino- 2-methyl phenol and 1-naphthol. The combination of 2,5-diamine toluene with the coupler 3-aminophenol gives a magenta-brown dye, while the combination of 2,5- diaminotoluene with the coupler 1-naphthol gives a purple dye.
- Yellow-green couplers include resorcinol, 4-chlororesorcinol, and benzodioxoles. These compounds produce broad-band absorption when they react to form dyes, allowing for more natural-looking hair colours. The combination of 2, 5-diaminotoluene with the coupler resorcinol gives a greenish-brown dye.
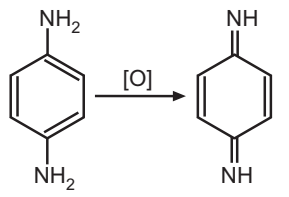
The first step shows the oxidation of p-phenylenediamine to the quinone diimine (C6H4(NH)2):
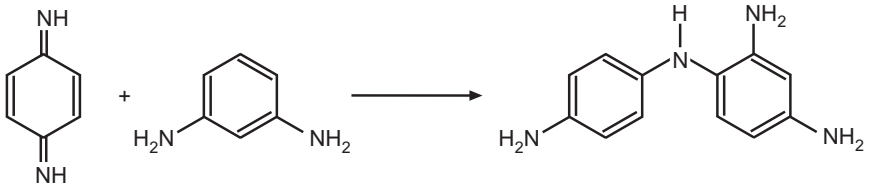
This species exists in equilibrium with the monoprotonated form (C6H4(NH)(NH2)) (not shown). The second step involves the attack of this quinone diimine on the coupler (electrophilic aromatic substitution)
In the third and final step, the product from the quinone diimine-coupler reaction oxidizes to the final hair dye.
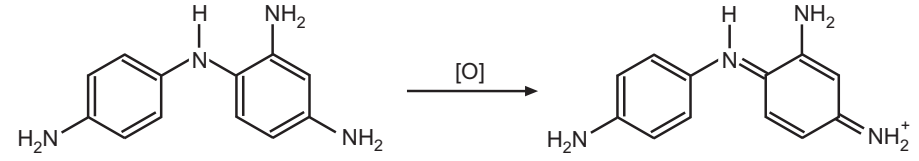
It was once believed that the dye forms in the above reaction bonds to hair permanently. It was later shown that the main reason that this reaction imparts a permanent colour on hair is by producing larger dye molecules, which is locked inside the hair.
Plant Dased Dyes
Henna is the most widely used vegetable dye for hair, promoting reddish-orange colour shades. In some commercial products, it is mixed with other dyes to increase the range of colour. It consists of the dried leaves of the Lawsonia alba plant, growing in North Africa, in the Midwest, and India. Its colouring properties are due to the presence of the substance 2-hydroxy-1,4-naphthoquinone, soluble in hot water and substantive to hair keratin in pH 5.5. It is therefore considered semi-permanent to permanent, depending on a person’s hair type. Most people will achieve a permanent colour from henna, especially after the second dye. With repeated use, the orange colour builds up into red and then auburn. While “natural” henna is generally a red colour, variations exist. These variations usually contain ingredients from other plants and even synthetic dyes. Using a plant-based colour such as henna can cause problems later when trying to do a perm or permanent hair colour. Some commercial formulations contain metallic salts which react to hydrogen peroxide that is used in hair lightening. This may lead to unpredictable results, such as green or blue tones in the hair. Henna is a healthy way to colour hair, as long as no metallic salts are used.
Indigo is a natural dye from a plant (Indigofera tinctoria, suffruticose, or arrecta) that can be added to henna or layered on top of it to create brown to black colours in the hair. Henna is orange, and indigo is blue, so as complementaries on a standard colour wheel, the two colours’ combined effect is to create brown tones. Like henna, indigo may fade after one application, but it becomes permanent on the hair with repeated use.
Another vegetable dye commonly used to obtain yellow shades is chamomile that promotes greater light reflection. Of all the species of chamomile, only Anthemis nobilis (Roman Chamomile) and Matricaria chamomilla (German chamomile) have cosmetic applications, and both are substantive to hair. The active ingredient of the flowers is 1,3,4-trihydroxyflavone, also known as apigenin.
Make sure you also check our other amazing Article on : Anti Dandruff Shampoo
