The hydrophilic-lipophilic balance (HLB) system is based on the concept that some molecules of surfactants are having hydrophilic groups; other molecules have lipophilic groups and some have both hydrophilic and lipophilic groups called amphiphilic molecules. Hydrophilic and lipophilic portions dissolve in an aqueous and oily phase. It is useful to correlate and measure these characteristics of the surfactants by some means for their applications in various fields such as to formulate various dispersed systems like lotions and emulsions. A common system, which is used to express the amphiphilic nature as a balance between a hydrophilic and lipophilic portion of the molecule is called as HLB system.
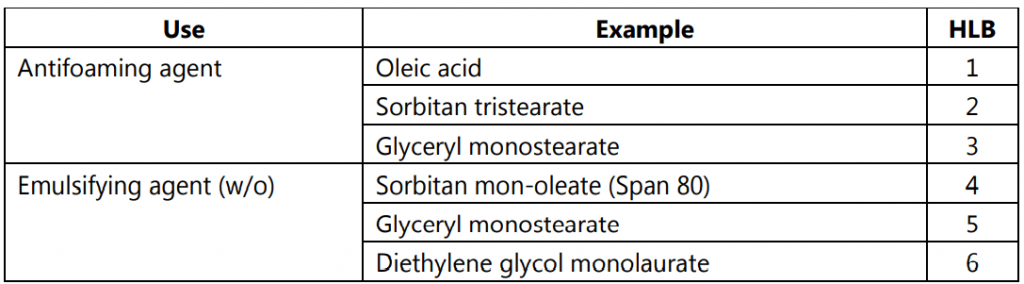
The weight percentage of each type of group in a surfactant molecule or a mixture of surfactants predicts what behavior the surfactant molecular structure will exhibit. Griffin in 1949 and its later development in 1954 introduced the HLB system, a semi-empirical method. It is the number on a scale of 1 to 40, as shown in Fig.1. The HLB value for a given surfactant is the relative degree to which the surfactant is water-soluble or oil soluble. An emulsifier having a low HLB number indicates that the number of hydrophilic groups present in the molecule is less and it has a lipophilic character. For example, spans generally have low HLB numbers and are also oil soluble. Because of their oil-soluble character, spans cause the oil phase to predominate and form a w/o emulsion.
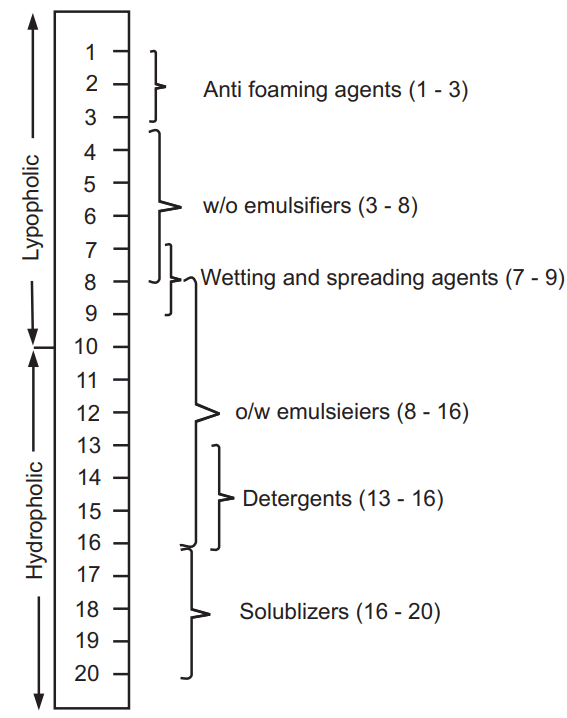
A higher HLB number indicates that the emulsifier has a large number of hydrophilic groups on the molecule and therefore is more hydrophilic. Tweens have higher HLB numbers and are also water-soluble. Because of their water-soluble character, Tweens will cause the water phase to predominate and form an o/w emulsion.
The usual HLB range is from 1 to 20, while there is one exception to this range as shown in Table.1 at the bottom. Sodium lauryl sulfate, a surfactant dissolves in water very well and is a common additive to most of the heterogeneous systems and almost all common detergents. As the HLB value is additive, the blending of surfactants with known HLB values to get the desired one is very easy. The appropriate HLB values are calculated by various methods.
Methods to Determine HLB:
Table of Contents
The method I – Alligation or Algebraic manipulations:
If a and b are the HLB values of surfactant A and B, respectively, and the c is desired HLB value then proportional parts required of A and B surfactants are x and y, respectively.
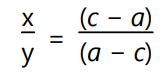
HLBBlend = f HLBA + (1 − f) HLBB equation (2)
where f is a fraction of surfactant A and (1 − f) is a fraction of surfactant B in the surfactant blend.
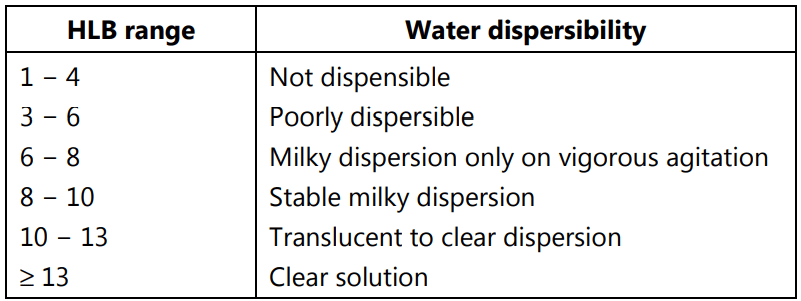
Method II – Water dispersibility:
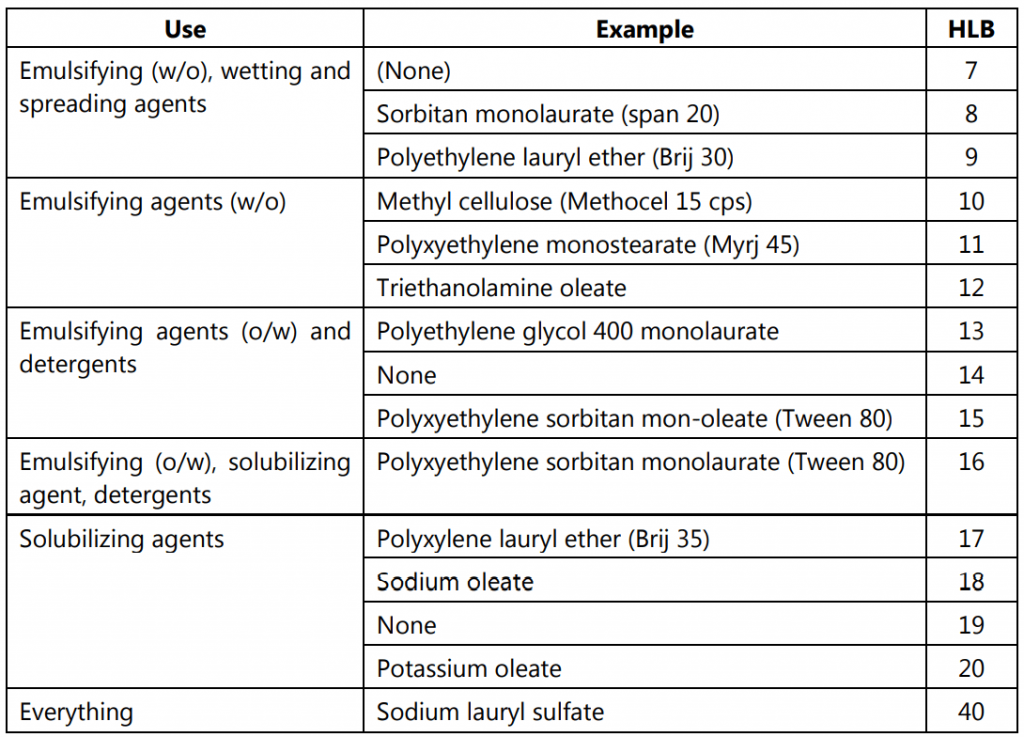
Approximation of HLB for those surfactants and not described by Griffin can be made either from the characterization of their water dispersibility, Table.2.
Method III – Experimental estimation:
From experimental estimations, blends of unknown surfactants in varying ratios with an emulsifier of known HLB are used to emulsify the oil. The blend that performs best is assumed to have a value approximately equal to the required HLB of the oil.
Method IV – Group contribution method:
Davis and Rideal suggested an empirical calculation of HLB based upon the positive and negative contribution of various functional groups to the overall hydrophilicity of a surfactant. Substituting values given in Table 3. for various group numbers in equation (3) gives HLB of a surfactant.
HLB = ∑ (Hydrophilic group number) − ∑ (Lipophilic group number) + 7 equation (3)
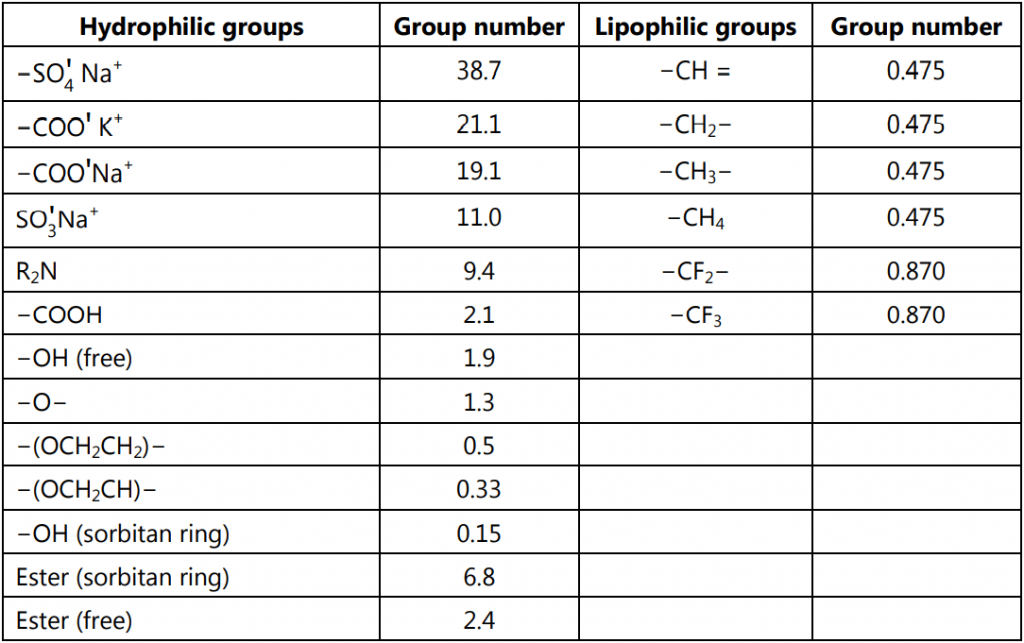
Experimental estimations of HLB values of lanolin derivatives like beeswax and wool fat cannot be obtained easily. Each atom or group has been assigned a constant and used in the calculation of HLB. For example, if surfactant contains Polyoxyethylene chains, the HLB is calculated by the equation;
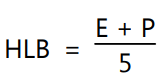
where, E and P are percents by weight of oxyethylene chains and polyhydric alcoholic groups, respectively in the surfactant molecule. When the molecule contains only oxyethylene groups then, HLB is calculated by the equation:
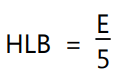
HLB values of surfactants such as glyceryl monostearate that contain fatty acid esters and polyhydric alcohols are calculated by the equation:

where S is saponification number and A is acid number.
Saponification number is defined as the number of milligrams of potassium hydroxide required to neutralize the acid formed during the saponification of one gram of sample. The acid number is the number of milligrams of potassium hydroxide required to neutralize the free acid in one gram of sample. One part of the structure of glyceryl monostearate contains a fatty acid stearic acid, which is lipophilic while the other part is alcohol, which is hydrophilic. Therefore, analysis of these parts by saponification gives HLB estimates.
Factors Affecting HLB Value:
1. Nature of immiscible phase
2. Presence of additive
3. Concentration of surfactant
4. Phase volume
5. Temperature
Drawbacks of the HLB system:
HLB system provides only information about the hydrophilic and lipophilic nature of the surfactants but the concentration of these surfactants is not considered. For optimum stability and therapeutic safety concentrations of the surfactant are equally important. It does not consider the effect of temperature as well as the presence of other additives.
Make sure you also check our other amazing Article on : Surface Active Agents