Introduction of Stereoisomerism: Stereochemistry helps to define the structure of a molecule and the orientation of the atoms and functional groups present, in three dimensions. Stereoisomers possess the same molecular and structural formulae and the same functional groups but differ in the three-dimensional spatial orientation of these atoms or groups within the molecule. Due to the difference in orientation of the functional group and geometry of the molecule, stereoisomers differ in their physical, chemical, physicochemical, and biochemical properties. Based on symmetry and energy criteria, stereoisomers are divided into three classes.
- (a) Geometrical isomers
- (b) Optical isomers
- (c) Conformational isomers.
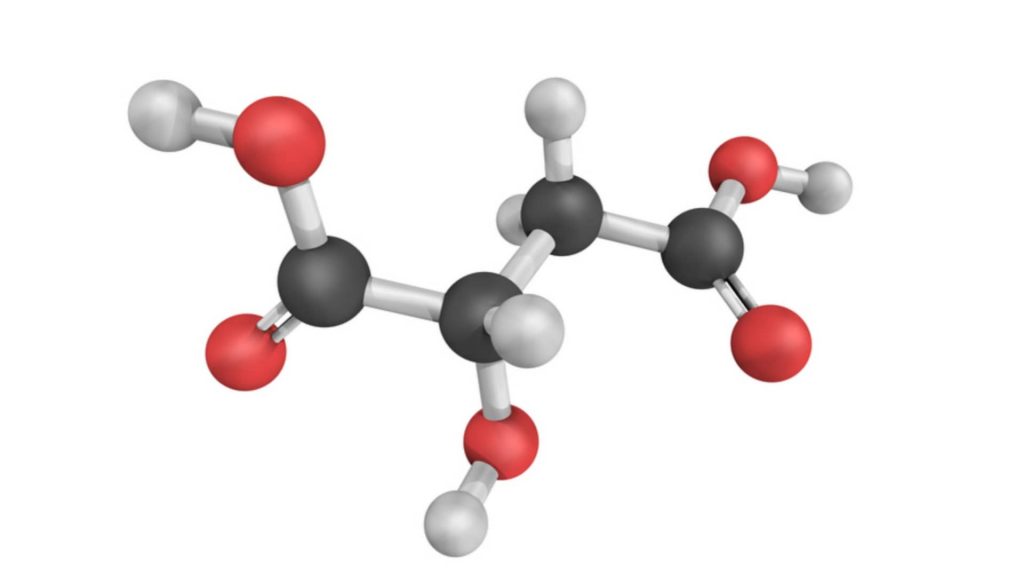
(a) Geometrical isomers (cis-trans isomerism): Maleic acid (m.p. 130°C) and fumaric acid (m.p. 287°C) have the same molecular formula but differ in the arrangement of functional groups around a double bond. They have different physical and, to some extent, chemical properties. This type of isomerism is known as geometrical isomerism.
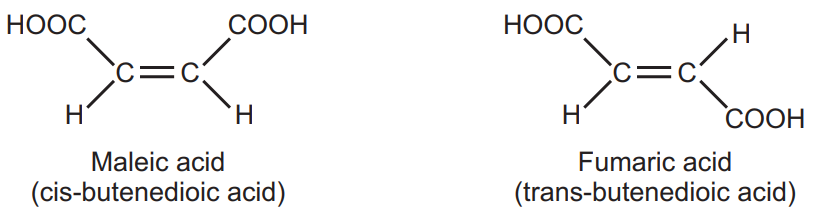
The presence of a carbon-carbon double bond restricts the freedom of rotation about the double bond. The designation cis (Latin word: same side), is used to denote the presence of like atoms or groups on the same side and trans (Latin word, across) is used when they are on opposite sides. Isomerism seen in non-cyclic, open-chain compound due to the presence of a double bond, is called π diastereoisomerism while when it occurs in a cyclic skeleton lacking a double bond, it is termed as σ diastereoisomerism.
(b) Optical Isomerism (enantiomerism): In 1815, Biot found that several organic and inorganic compounds in the solution form can rotate the plane of polarized light in opposite directions but in identical amplitude, passing through them. Optical isomerism is seen in compounds that can rotate plane polarised light. A carbon atom connected to four chemically different functional groups is known as asymmetric or chiral carbon and the presence of at least one asymmetric carbon atom in the structure is the prerequisite for a molecule to show optical isomerism.
If there is one asymmetric carbon then two optically active isomers are possible. Isomer rotating plane of polarized light to the right is said to be dextrorotatory (Latin, dexter: right) while isomer showing rotation to the left is known as laevorotatory (Latin, leaves: left). Both isomers are mirror images of each other yet are not superimposable. They are called enantiomers and the pair of enantiomers is called enantiomorphs. An enantiomer does not possess a plane or center of symmetry. For example,

When the enantiomers are present together in equal concentration, the rotation of plane-polarized light caused by laevo isomer will be neutralized by a dextro rotating isomer and the mixture will be optically inactive. Such mixtures are called racemic mixtures. The conversion of an enantiomer into a racemic form is called racemization. While the separation of a racemic mixture into individual enantiomers is called resolution. The maximum number of optically active isomers possible for a molecule having more than one asymmetric carbon atom may be given by the formula
N = 2n
where,
N = Number of optically active isomers, and
n = Number of asymmetric carbon atoms.
Except for rotation of plane-polarised light, enantiomers have identical physical and chemical properties like boiling point, melting point, solubility. Their chemical properties are the same as achiral reagents, solvents, and conditions. Towards chiral reagents, solvents, and catalysts, enantiomers react at different rates.
As per the rule given above, tartaric acid will have four optically active forms because of the presence of two asymmetric carbon atoms.
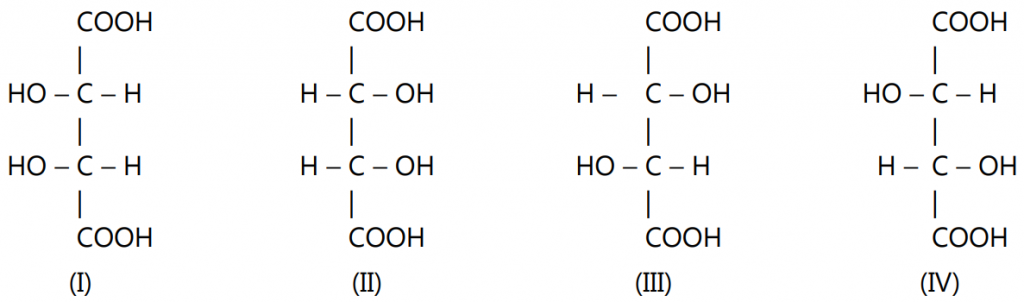
Forms (I) and (II) are identical and symmetrical. In these forms, the upper half is the mirror image of the lower half. This makes the molecule optically inactive through internal compensation. Such identical and symmetrical stereoisomers are called meso-isomers.
Forms (III) and (IV) are mirror images of each other but are not superimposable. They are enantiomeric forms.
While if you compare (III) with (I) or (IV) with (I), these are not enantiomeric pairs. They are neither mirror images nor superimposable. Only one of the two halves of their molecules is identical while the remaining halves are mirror images. Such stereoisomers which do not mirror images and are non-superimposable are called diastereomers. They have different physical and chemical properties, with both achiral and chiral reagents. The rates are different and the product may be different.
Make sure you also check our other amazing Article on : Quality Control of Aerosols