Introduction:
Table of Contents
Investigational New Drug (IND) is a program by which any pharmaceutical company can approach to obtain permission for the initiation of human clinical trials and to ship an experimental drug across state lines (usually to clinical investigators) before a marketing application for the drug has been approved.
- An investigational new drug (IND) application is to provide the data showing that it is reasonable to begin tests of a new drug on humans.
- The IND application is also the vehicle through which a sponsor advances to the next stage of drug development known as clinical trials.
- Current Federal law requires that a drug be the subject of an approved marketing application before it is transported or distributed across state lines (Clinical Investigators).
- Because a sponsor will probably want to ship the investigational drug to clinical investigators in many states, it must seek an exemption from that legal requirement.
- The IND application is the means through which the sponsor technically obtains this exemption from the FDA.
- During a new drug’s early preclinical development, the sponsor’s primary goal is to determine if the product is reasonably safe for initial use in humans and if the compound exhibits pharmacological activity that justifies commercial development.
- When a product is identified as a viable candidate for further development, the sponsor then focuses on collecting the data and information necessary to establish that the product will not expose humans to unreasonable risks when used in limited, early-stage clinical studies.
- FDA’s role in the development of a new drug begins when the drug’s sponsor (usually the manufacturer or potential marketer), having screened the new molecule for pharmacological activity and acute toxicity potential in animals, wants to test its diagnostic or therapeutic potential in humans.
- At that point, the molecule changes in legal status under the Federal Food, Drug, and Cosmetic Act and becomes a new drug subject to specific requirements of the drug regulatory system.
Classification of IND
Commercial: Permits sponsor to collect data on clinical safety and effectiveness needed for application for marketing in the form of NDA.
Research (Non-commercial): Permits the sponsor to use the drug in research to obtain advanced scientific knowledge of the new drug. No plan to market the product.
TYPES OF IND APPLICATIONS
- Investigator IND application
- Emergency Use IND application
- Treatment IND application
- Screening IND application

1. Investigator IND Application:
In this, an application is submitted by a physician, who both initiates and conducts an investigation, and under whose immediate direction the investigational drug is administered or dispensed. A physician might submit a research IND application to propose studying of:
- An unapproved drug,
- An approved product for a new indication or
- An approved product in a new patient population.
2. Emergency Use IND Application:
This application allows the FDA to authorize the use of an experimental drug in an emergency that does not allow time for submission of an IND application, following 21CFR, Sec. 312.23 or Sec. 312.20. It is also used for patients who do not meet the criteria of an existing study protocol, or if an approved study protocol does not exist. In such a case, FDA may authorize the shipment of the drug for a specified use in advance of submission of an IND application.
3. Treatment IND Application:
- This application is submitted for experimental drugs showing promise in clinical testing for serious or immediately life-threatening conditions while the final clinical work is conducted and the FDA review takes place.
- A drug that is not approved for marketing may be under clinical investigation for a serious or immediately life-threatening disease condition in patients for whom no comparable or satisfactory alternative drug or other therapy is available.
- In the case of a serious disease, a drug ordinarily may be made available for treatment use during phase III investigations or after all clinical trials have been completed.
- In the case of an immediately life-threatening disease, a drug may be made available for treatment use earlier than phase III, but ordinarily not earlier than phase II.
4. Screening IND Application:
It is filed for multiple, closely related compounds to screen for the preferred compounds or formulations. The preferred compound can be developed under a separate IND. It can also be used for screening different salts, esters, and other drug derivatives that are chemically different, but pharmacodynamically similar.
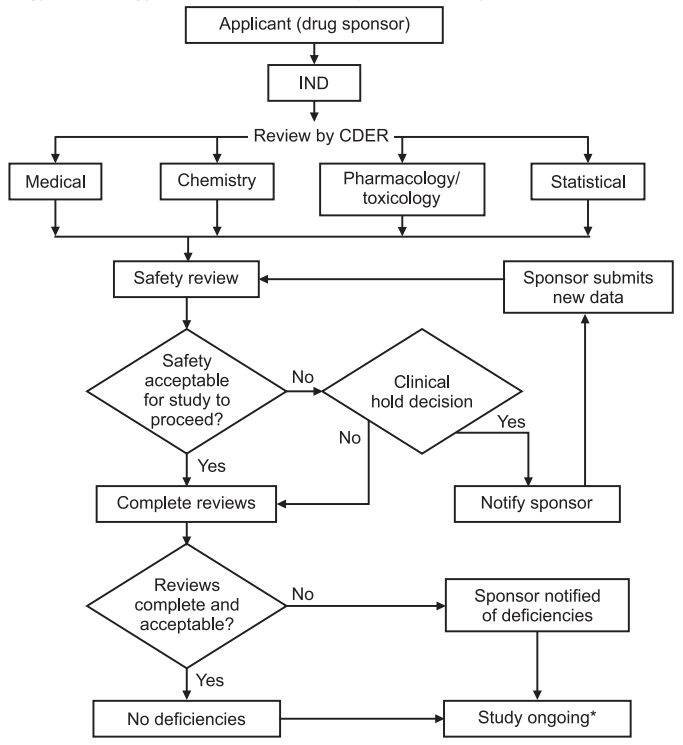
IND Review and Report: During this time, FDA has an opportunity to review the IND application for safety to assure that research subjects will not be subjected to unreasonable risk. The report evaluates the various factors like Medical Review, Chemistry Review, Pharmacology/Toxicology review, Statistical analysis, and Safety review.
Make sure you also check our other amazing Article on : Regulatory Requirements for Drug Approval