Investigational drugs are those drugs or mixtures of chemicals which are not released and certified by the food and drug administration (FDA) for the general use and sale for the commercial concern. These drugs usually stand the statement on their labels as
“Caution: New Drug – Limited by Federal Law to Investigational use”. They are released only to the principal investigator who is a member of the medical staff of the hospital after obtaining consent by duly signing the food and drug release form for the manufacture of respective investigational drugs.
The clinical pharmacists have a deliberate position because the evaluation program tries in the hospital setting where the required laboratory and medical facilities are available for the procedure of investigational drugs use.
Table.1: Classification of Investigational New Drugs
| Class A | • This class should contain all investigational use drugs which are under the preliminary experimental stage. • These drugs are restricted to use only by the principal investigator. |
| Class B | • This class of investigational drugs has passed through the preliminary experimental research stage. In this class, the investigational drugs are supplied to the pharmacy department by the principal investigator and are only dispensed after obtaining his written prescription which is duly signed. |
| Class C | • This class of investigational drugs is approved by the USP, NF, or passed by the Federal FDA for use and sale as a commercial concern. These drugs may be used only in hospital setup for their patients under the supervision of medical staff after fulfillment of a specific procedure |
| Class D | • These classes of drugs have been accepted for use in the hospital and are listed in the hospital formulary. |
Statement of Principles Involved in The Use of Investigational Drugs
Table of Contents
The investigational drug should be used only in a hospital setup, which is the primary center for clinical investigations. As per the definition, these are not general-purpose use and these are not yet to release or certified by FDA for general use or sale in commercial interest. It is the responsibility of the hospital and medical staff to check and establish the proper procedure for the use of investigational drugs for their patient’s benefit. The procedure and the control on the use of investigational drugs are based on the following principles:
1. The investigational drugs should be used under strict medical supervision, mainly under the supervision of a principal investigator who is supposed to be a member of medical staff and has the responsibility of obtaining necessary consent.
2. Hospitals should do these procedures for short-term research purposes in most critical cases such as; advanced stage of cancer with the aim at beneficial and protection for their patients.
3. When the nurse is called for drug administration and is a part of the procedure, then the nurse should have detailed information of investigational drug such as; dosage form, available strength, the action of the drug, its uses, side effects, and toxicities, etc.
4. It is the responsibility of the hospital to establish a central unit with the help of the pharmacy and therapeutics committee for the maintenance and availability of essential information on the investigation drugs to authorized personnel.
5. Pharmacy department should find an appropriate place for the storage of such investigation drugs. The department will also be responsible for the provision of proper labeling and dispensing by the written order of the principal investigator.
Advisory Committee and their Responsibilities:
In the view of drugs which are used in the investigational purpose in the hospital are subjected to review by advisory committees which are nothing but the committee on the human use in research; and Pharmacy and Therapeutics Committee (PTC). In which the principal investigator should provide all the information related to investigational drugs to the PTC and should letter for intention to use of investigation drugs in patients. It is the responsibility of the hospital and members of PTC to develop procedures and policies for the handling of investigational drugs in the hospital for the patient’s benefit.
The committee on human use in clinical research is the standing committee of the hospital and is responsible for providing the guiding principles and policies issues in association with the use of humans/patients for the clinical research investigations.
Following are some important policies which are needed to be implemented in the hospital where enforcing the use of investigational drugs safeguard the rights and welfare of human subjects.
- The use of an investigational drug in patients must be approved by the human subject committee, pharmacy, and therapeutics committee, and if applicable isotope committee before the use for the patients in the hospital.
- The principal investigator shall be informed in the written that neither the hospital nor other hospital staff will be responsible for any legal liabilities which may occur during the use of investigational drugs.
- The investigator should not proceed to use the investigational drug before obtaining the consent form from the patient or legally responsible person.
- The principal investigator is responsible for registering each investigational drug in the pharmacy department and providing all the related information to prepare an investigational drug data sheet and drug formulary.
- To ensure the continuous observation of the research project by the committee of human use in research, each principal investigator should prepare a continuing surveillance report every quarter and forward it to the secretary of the committee of the use of humans in research investigations regularly.
Control and Supervision of Investigational Use of Drugs
All investigational drugs which are in use, are under the strict supervision of the principal investigator and are registered with the Pharmacy and Therapeutics Committee (PTC). The following information must be given by the principal investigator to PTC with a letter indicating the intention to use such investigational drug in a patient under his supervision.
- New drug number
- Generic name
- Manufacturer
- Chemical name
- Proprietary name
- General chemistry
- Pharmacology
- Toxicology
- Dose range
- Method of administration
- Antidote
- Therapeutic use
The above information on each investigational drug is usually found in the brochure which is provided by the manufacturer and supplier to the principal investigator after fulfillment of the official procedure.
The pharmacy department may use this information in the form of a Physician Data Sheet on the Investigation drugs for circulation to various medical staff and nurses dealing with the use of particular investigational drugs.
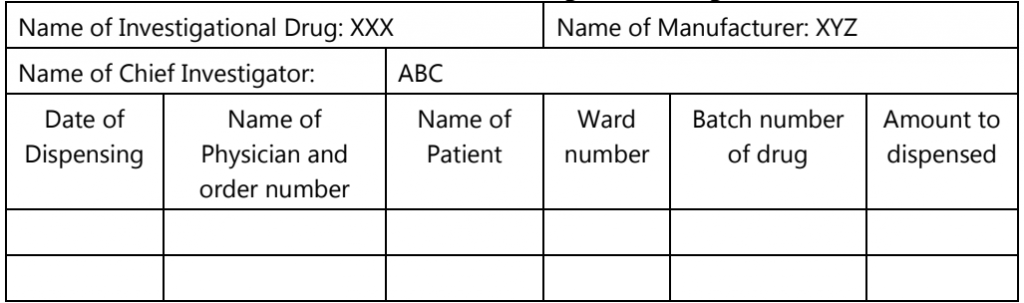
Hospitals can design drug report forms for use of investigational drugs. Such form may provide all information required for the handling and dispensing procedure for the investigational drug by the medical staff, nurse, and pharmacist. This form must be kept in such a place where it can easily be accessible to staff associated with the handling of investigational drugs. Each staff dealing with the procedure of investigating drug use must comply with the guidelines frame by the hospital and maintain the record in duplicate entries. Similarly, pharmacists should maintain records (separate files) for the dispensing of each investigational drug along with receipt of written prescriptions of principal investigators.
Table.2: Layout of Data Sheet for Dispensing Record Maintenance of Investigational Drug
Identification of Investigational Use of Drugs
Pharmacists must ensure to label each class of investigational drugs before they are going to dispense. Labeling of class A and class B drugs in such a manner that, these class drugs should be differentiated from routine prescription drugs. In some hospitals, for proper differentiating with other classes, hospitals use printed labels in red ink on the white paper stock. In addition to this label information, the hospital also provides the above-printed layout format for the dispensing record of the investigational drugs
Role of Hospital Pharmacist in Handling of Investigational Drugs
1. Assisting in the development of the study design: Pharmacists provide support in the development of the new protocol and control on the use of investigational drugs in the hospital. The investigational drugs are assigned to the pharmacist for dispensing purposes according to a written order from the principal investigator in a predetermined pattern.
2. Acting as Independent Collaborator: The pharmacist act as an independent collaborator by maintaining all the records and codes in the handling of investigational drugs in the hospital. This would give the investigators the advantages of having code information available 24 hours a day and 7 days a week and the ability to break the code for an individual patient without exposing the rest of the study.
3. Collecting, Storing, and Distributing Essential Information Concerning the Investigational Drug Being Studied: As per the brochure provided by the manufacturer, the pharmacist should prepare a data sheet on the investigational drug which provides the all information about the investigational drugs. These drug data sheets give information to the medical, pharmacy, and nursing staff. This form should contain:
- Drug designation and common symptoms.
- Dosage form and strengths available.
- Dosage schedule and route of administration.
- Indicators.
- Expected therapeutic effects.
- Expected and potential untoward effects.
- Contraindications.
- Storage requirements.
- Instructions for dosage preparation and administration.
- Instructions for disposition of unused doses.
- Names and Telephone numbers of principal and authorized co-investigators.
The data sheet on the investigational drug is reviewed by the principal investigator and then the copies are distributed to the appropriate pharmacy staff and all the patient care units whenever the drug will be used.
4. Packaging and Labelling of Investigational Drugs for Multiple or Unit Dose Containers: Investigational drugs must be properly packaged by all applicable regulatory standards for example: F. D. A, C.P. Packaging act.
5. Preparing Dosage Forms: The pharmacist can provide a valuable service to the new drug researcher by formulating a new dosage form from the pure chemical.
6. Dispensing of Investigational Drugs to both Inpatients and Outpatients: Dispensing of investigational drugs should be incorporated with the rest of the drug distribution system. The pharmacist should maintain the inventory record for the dispensing of investigational drugs. This form should contain the name of the drug, dosage form, and strength, batch number, and name of the manufacturer along with a complete address. In this form, there is a need to maintain the record of other information which are essential for the drug order. It is essential to provide sufficient information on proper dosage, route of administration, possible toxic reactions and side effects, precautions and proper labeling is available to them.
Make sure you also check our other amazing Article on : Clinical Pharmacy
