Lithium Methoxide (0.1M)
Molecular formula: LiOCH3
Molecular weight: 37.97
Preparation: Dissolve in a small portion of 0.7 g of freshly cut lithium metal under cool conditions in 100 mL of methanol. After completion of reaction add 850 mL of toluene. Add a sufficient amount of methanol to get a clear solution of 1000 mL. Store the solution in a container well protected from carbon dioxide and moisture.
Standardization: Weigh accurately about 0.25 g of benzoic acid, dissolve in 25 mL of dimethyl-formamide, add 0.1 mL thymol blue in dimethyl formamide as an indicator and titrate with lithium methoxide solution to give blue color as the endpoint. Perform a blank determination and make any necessary corrections.
Reaction:
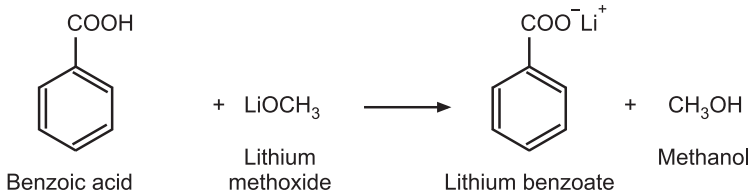
Factor Calculation:
1 mole of LiOCH3 ≅ 1 mole of benzoic acid
1000 mL of 1M LiOCH3 ≅ 122.12 g of C6H6COOH
1 mL of 0.1 M LiOCH3 ≅ 0.01221 g of C6H6COOH
Make sure you also check our other amazing Article on: How is nitric acid prepared?
