Measurement of Bacterial Growth: Measurement of microbial growth and physiology is an important parameter of microbial study. This measurement to a great extent depends on the number of microorganisms present in a culture. The growth of microorganisms can be quantitatively measured using various techniques. Microbial growth can be measured either by colony counting or cell counting, by weighing the cell (cell mass measurement), or by cell activity measurement.
1. Determination of cell number:
(a) Total count/ direct methods:
- Direct microscopic count/ Breed method.
- Counting chamber method/ Haemocytometer method.
- Proportional count method.
- Electronic counter method.
(b) Viable count/ indirect method:
- Plate count technique.
- Membrane filter count.
2. Determination of cell mass:
(a) Direct method:
- Dry weight measurement
- Measurement of cell nitrogen.
(b) Indirect method:
- Turbidimetric method.
3. Determination of cell activity:
- Measurement of biochemical activity (indirect method).
1. Determination of cell number
(a) Total count or direct methods:
The total count of micro-organisms in any given suspension indicates the total number of cells which includes both living and dead. The following methods are used for the determination of the total count.
(i) Breed method: This method is also called ‘Direct microscopic count’. A known volume of cell suspension (0.01 ml) is spread uniformly over a glass slide within a specific area (1 sq. cm). The smear is then fixed, stained, and examined under the oil immersion lens, and the cells are counted. It is impossible to examine the entire area (1 sq. cm), therefore practically only a few microscopic areas are observed. Several microscopic fields are counted and an average is taken. The total cells per square cm are then calculated by determining the number of microscopic fields per square cm.
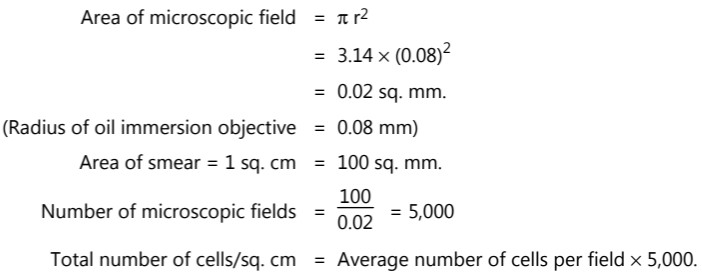
(ii) Counting chamber method: Bacteria can be counted easily and accurately with the Petroff – Hausser counting chamber or Haemocytometer. Counting of microorganisms can be made rapidly and simply with minimum equipments. In the hemocytometer or counting chamber method, a minute drop of the culture is placed in a tiny, shallow, rectangular glass slide called Neubar’s slide. This is a special slide accurately ruled into squares that are 1/400 mm2 in area. A suspension of unstained bacteria can be counted in the chamber, using a phase contrast microscope.
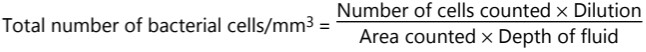
Since cells are counted in five bigger squares and such a square is further divided into 16 small squares.
(iii) Proportional count method: A standard suspension of particles (plastic beads, number of particles/volume is known) is mixed with an equal amount of cell suspension. This mixed suspension is spread on the slide, fixed, and stained. The particles and cells in the microscopic field are counted. An average count of the particles and the cell is taken from the number of fields.
e.g. Suppose an average count of 10 particles and 50 cells per field is obtained. If the number of particles in 1 ml of standard suspension is 25,000.
Then the number of cells/ml of suspension is:
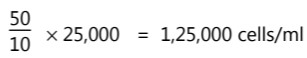
(iv) Electronic counter method: An electronic instrument, a Coulter counter can be used for the direct enumeration of cells in a suspension. In this technique, the bacterial suspension is passed through a capillary tube. The diameter of this tube is so microscopic that it allows only one cell to pass at a time. The instrument can count thousands of cells in a few seconds. But this system has a disadvantage, in that the Coulter counter counts even dust particles. Hence, the suspension must be free of any foreign particles.
Direct counting methods are rapid and simple. The morphology of cells can also be observed when they are counted under the microscope. The major disadvantage of this method is that it gives the total cell count which includes both viable and non-viable cells. Accuracy also declines with very dense and very dilute suspensions because of clumping and statistical errors, respectively.
(b) Viable count/Indirect method:
The principle behind viable count is that all viable cells or spores, under suitable growth conditions multiply and that each cell or spore forms a colony. The number of colonies, therefore, is the same as the number of viable cells present in the original sample.
(i) Plate count technique: In this method, a measured amount of diluted bacterial suspension is introduced into a Petri plate, after which the agar medium (liquid form 45o C) is added. (Fig.1). Immediately, mix the agar medium with the inoculum by rotating the plate. After the solidification of the medium, the plates are incubated at 37o C for 24 hours in an inverted position. A plate having 30 to 300 colonies is selected for counting the number of microorganisms.
The plate count technique has certain disadvantages. If the suspension contains different microbial species, then all of them may not grow on the medium used and under the specified conditions of growth. If the suspension is not homogenous and contains aggregates of cells, the resulting colony count will be lower than the actual number of microorganisms because aggregate cells produce a single colony.
The plate count technique is used routinely for the estimation of bacterial populations in milk, water, foods, and other materials. The method is highly sensitive, i.e. extremely high or very low viable populations can be counted.
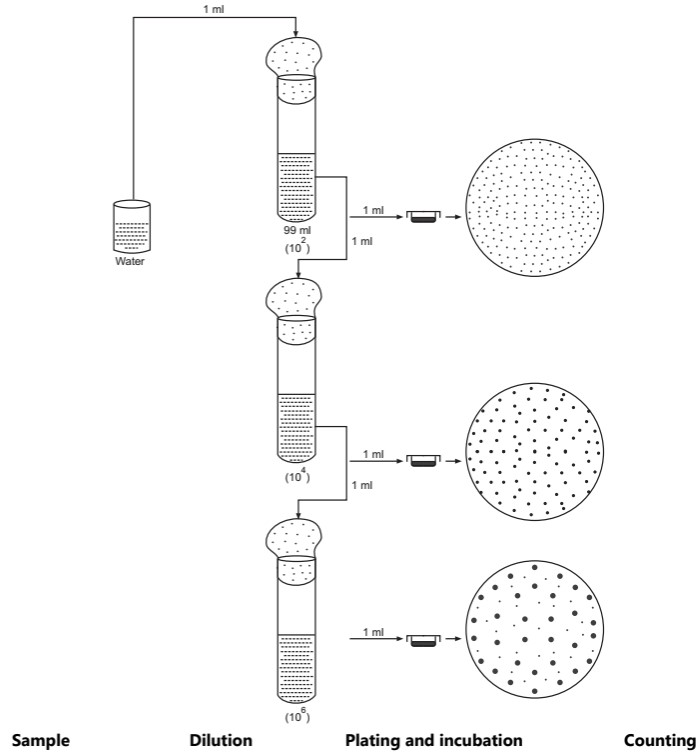
(ii) Membrane filter count: This method follows the same principle as in a plate count technique. A diluted suspension of microorganisms is filtered through a millipore filter. The microbes are retained on the filter disc and this disc is placed in a culture medium on a Petri plate. The plates are incubated and the colonies are counted on the filter disc.
The membrane filter method has many advantages over the plate count. A large volume of the sample can be analyzed, especially when the number of organisms is very few. Secondly, various types of microorganisms can be detected by using selective media in the plates and under different conditions of growth.
2. Determination of cell mass
This is a method in which, the weight or mass of the cells is estimated as an indicator of increased growth.
(a) Direct method:
(i) Dry weight measurement: This is a simple and direct method of measuring cell mass. The culture suspension is centrifuged and the pellet is repeatedly washed to remove all foreign particles. The residue is then dried and weighed. This method is mainly applicable in research investigation and for measuring the growth of molds.
(ii) Measurement of cell nitrogen: A major chemical constituent of the cell is a protein of which nitrogen is an important ingredient. A bacterial population or cell crop can be measured in terms of cell nitrogen. The cells are obtained by centrifugation as mentioned in dry weight measurement and the cell nitrogen is estimated by chemical analysis. This method is useful for dense cell suspensions.
(b) Indirect method:
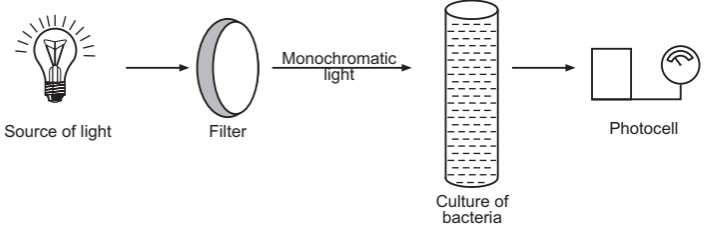
(i) Turbidimetric method: A most widely used technique for measuring cell mass is by observing the light scattering capacity of the sample. A suspension of unicellular organisms is placed in a colorimeter or spectrophotometer and light is passed through it (Fig.2). This instrument works on Beer and Lambert law i.e. light absorbance is directly proportional to the turbidity of the medium. The absorbance is measured in terms of optical density (OD). The measurement of optical density does not give the value of cell numbers or cell mass but the cell number can be calculated by plotting the ‘calibration curve’ which indicates, the direct relationship of optical density and mass. The cell number of an unknown sample can be determined by taking the optical density and comparing it with the corresponding value on the standard curve.
Turbidimetry is a simple and rapid method used for the determination of bacterial growth. However, this method is very accurate only with moderate density. It is not possible to measure cultures grown in colored media or cultures that contain suspended material other than bacteria. It must be recognized that dead as well as living cells contribute to turbidity. Applications of different growth measurement methods are given in Table.1.
3. Determination of cell activity
Measurement of biochemical activity: This is an indirect method to determine microbial growth. Measurement of a specific chemical change by the metabolic activities of microbes can be correlated with microbial growth. Cell metabolic activity results in the formation of any specific metabolite e.g. lactic acid, H2S, CO2, enzymes, etc. The measurement of these products forms the principle of measurement of cell activity. The amount of acid produced is proportional to the magnitude of cell suspension.
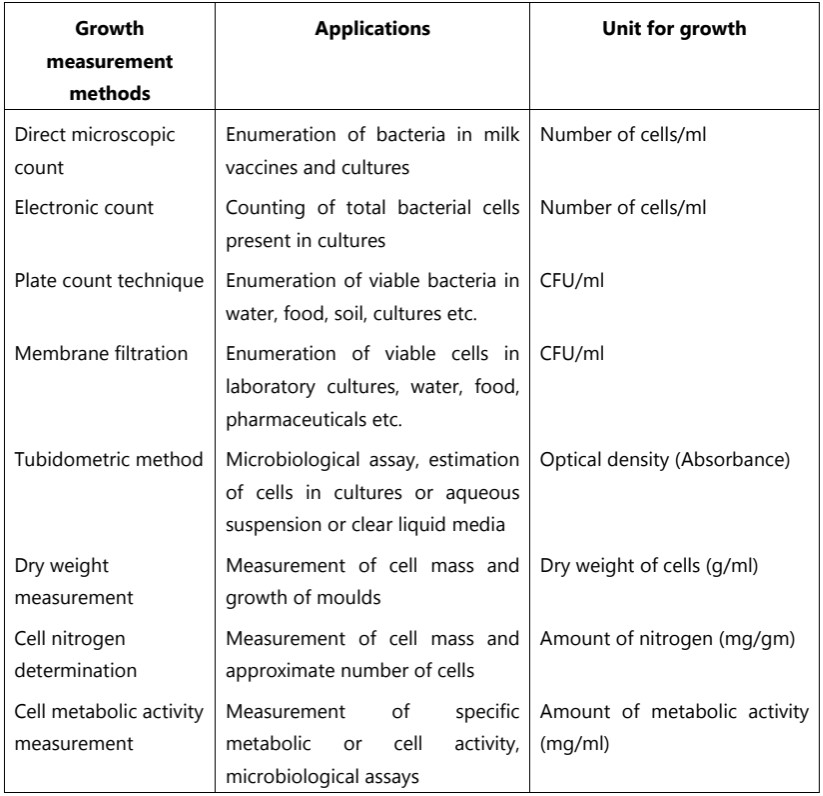
Table.1: Applications of bacterial growth measurement methods
Make sure you also check our other amazing Article on : Growth Curve of Bacteria