Mentha (Mentha Oil): Mentha species, one of the world’s oldest and most popular herbs, are widely used in cooking, in cosmetics, and as an alternative or complementary therapy, mainly for the treatment of gastrointestinal disorders like flatulence, indigestion, nausea, vomiting, anorexia, and ulcerative colitis.
- Synonyms: Peppermint oil, Oleum mentha Piperita, Mint oil.
- Biological source: Mentha oil is obtained by steam distillation of flowering tops of Mentha piperita Linn.
- Family: Labiatae.
- Geographical source: It is cultivated in Japan, England, France, Italy, the USA, Bulgaria, USSR and India (Jammu and Uttar Pradesh).
Cultivation and Collection of Mentha
Table of Contents
The basic requirement for the cultivation of mentha is well-drained fertile sandy loam soil (pH = 7), rainfall (95 to 105 cm), temperature (15 to 25°C), altitude (250 to 400 meters). The suckers are vegetatively propagated for cultivation purposes. The use of fungicides like mercury compounds enhances the better sprouting. The suckers are placed in the field in January or February month by keeping a distance of 10 cm. The distance between rows should be 50 to 60 cm. The foliar spray of urea and other fertilizers like superphosphate or potash is found advantageous. The harvesting should be done when plants reach the flowering stage. Thiodan (1: 700), endrine (1: 700), BHC (10 per cent), Sulphur (0.5 per cent) and malathion (5 per cent) are used for pest control.
Production of Mentha Oil
The plants are dried in the air. Sun-drying causes the loss of active principles i.e. volatile oils. The air-dried material is kept in an iron or steel vessel that has a false perforated bottom. The steam under pressure is passed through the drug for about 3 to 4 hours for complete distillation. Near about 80 per cent of oil is distilled off in the first half of distillation. The medicinally important menthol is distilled out in the latter half of distillation. The mentha oil is collected in a separating vessel where it is decanted and filtered. The average yield of oil is approximately 0.5 to 1 per cent v/w.

Description of Mentha plant
- Colour: Yellowish or colourless
- Odour: Agreeable, pleasant and characteristic
- Taste: Aromatic, Pungent (followed by cooling sensation)
- Solubility: Soluble in alcohol (70 percent), alcohol, ether, and chloroform insoluble in water.
- Weight per ml: 0.9 to 0.912 gm.
- Optical rotation: At 25°C −16° to −30°.
- Refractive index: 1.4590 to 1.4650.
- Chemical nature: Neutral to litmus paper.
Chemical Constituents of Mentha
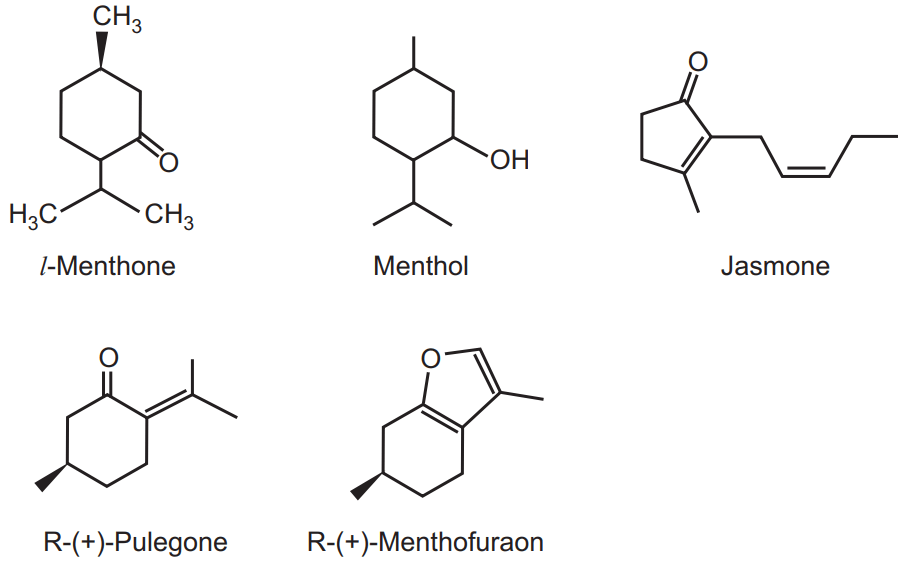
It mainly contains l-menthol (either in free form or ester form). There are three varieties of peppermint i.e. American variety (contain 80 per cent menthol), Japanese variety (contain 70 to 90 per cent menthol) and Indian variety which contain 70 per cent of menthol. Other important constituents are menthofuran, menthone, menthyl acetate, menthyl isovalerate, jasmone, and other derivatives like cineole, l-limonene, iso pulegone, camphene, pinene, jasmine, and esters (possess pleasant odour). Menthofuran is responsible for bad odour due to resinification.
Chemical Test of Mentha
Take a few ml of oil and mix it with 5 ml nitric acid solution. The solution should be made by adding 1 ml nitric acid into 300 ml of glacial acetic acid. Heat the solution in a water bath for 5 to 10 minutes. The liquid develops bluish colour which gets deep in colour upon more heating and shows copper coloured fluorescence and after a few minutes, it turns golden yellow.
Uses of Mentha
The oil is used as a carminative, stimulant, flavouring agent and antiseptic. It is used in pharmaceutical preparations, cosmetic industries, toothpaste, tooth powder, shaving cream, chewing gum, candies, jellies, perfumery and essence manufacturing.
The other therapeutical uses of mentha oil areas spasmolytic, smooth muscle relaxant, digestant, anti-inflammatory, anti-ulcer, nasal decongestant, antitussive etc. It is used in the preparation of topical preparation and lozenges.
The oil should be stored in the well-closed airtight umber coloured container because it becomes dark and viscous upon storage.
Make sure you also check our other amazing Article on : Dioscorea
