Molecular Distillation is a distillation process in which molecules travel a mean free path without coming into collision with one another by applying vacuum and also condensed separately.
Principle:
Table of Contents
- The mean free path is defined as the average distance traveled by single molecules in a straight line without any collision. It is expressed as
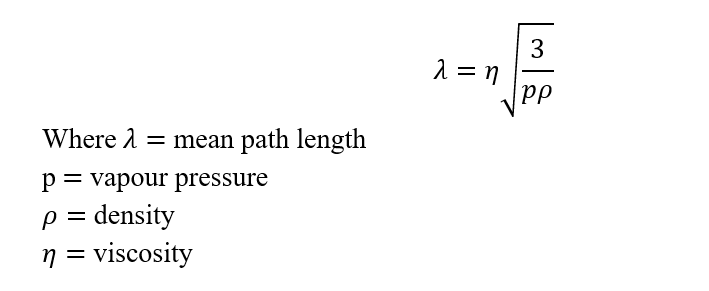
- Distillation is simple if liquids having low viscosity and density and also have a long mean path. There should be a minimum distance between the evaporating surface and condensing surface so that molecules pass through the condenser as soon as they leave the evaporating surface. Therefore molecular distillation is also called short path distillation. The intermolecular distance should be high which can be achieved by a very high vacuum (0.1 to 1.0 pascal)
Apparatus:
Falling film Molecular still or wiped film Molecular still:
Principle:
- The liquid film on the heated surface under high vacuum produces vapors and then each molecule condensed individually and distillate is collected.
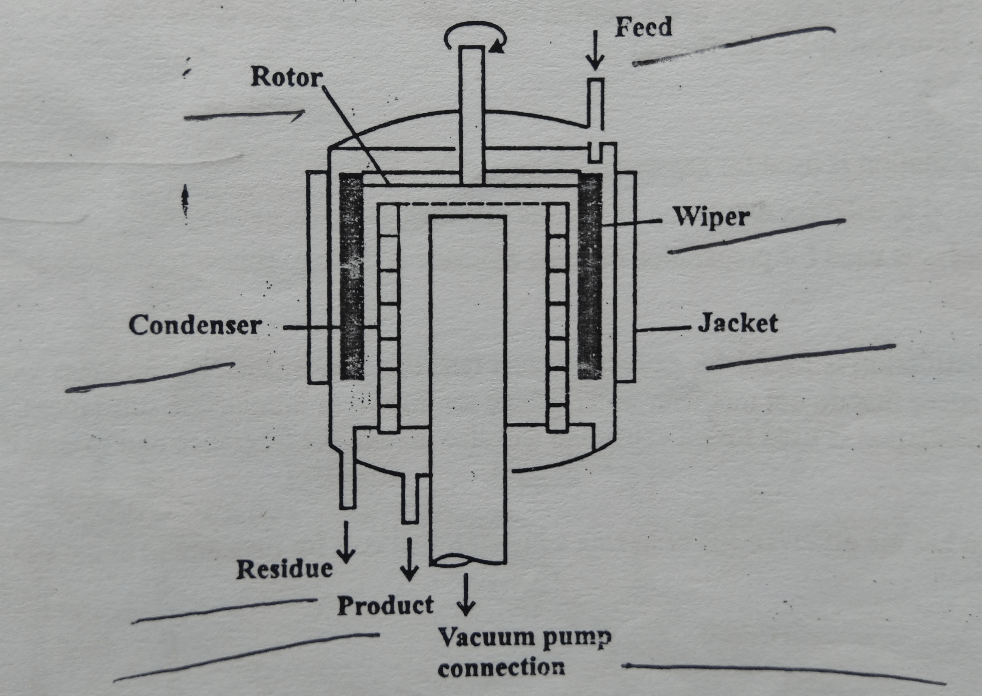
Construction of Molecular Distillation:
- It consists of a vessel (1 meter in diameter) and the jacket is provided around the vessel. The wiper is also attached to the wall of the vessel. Wipers are also attached to the rotating head through the rotor. Vacuum pumps are connected by a large diameter pipe. The condenser is also attached to the wall. There is also provision are made for feed (at top) and collecting the distillate and undistilled liquid residue (at the bottom)
Working of Molecular Distillation:
- The vessel walls are heated suitably by a heating jacket. The feed flows down the walls and is spread to a film by the polytetrafluoroethylene (PTFE) wipers which move about 3 m/s giving a film velocity of about 5 ms. Due to heat liquid film evaporates and vapors hit the condenser. Collect the condensate as a product The residue is collected at the bottom of the vessel and it is re-circulated (through the feed line).
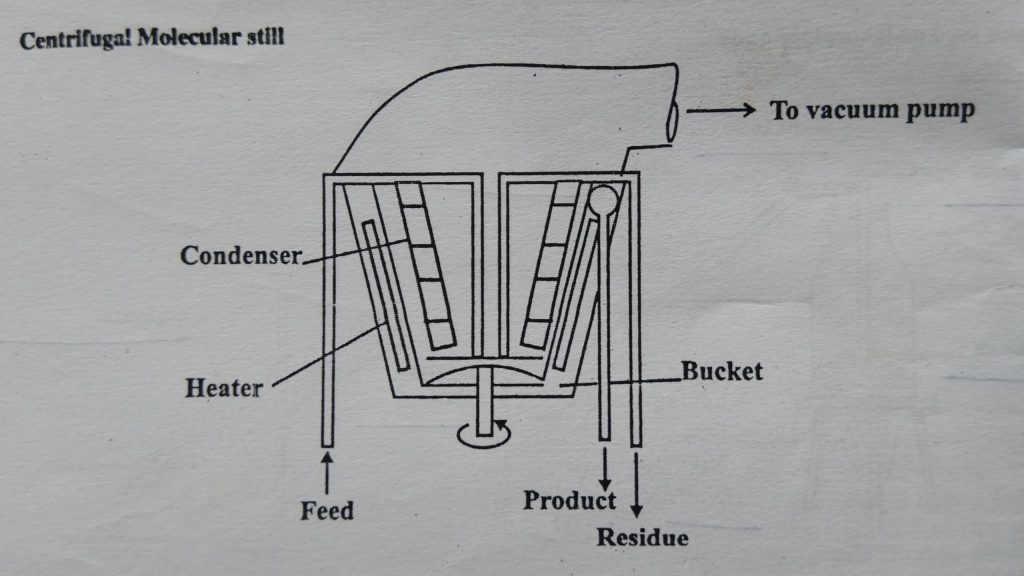
- The feed is introduced into the center of a bucket-shaped vessel (1 to 1.5 m in diameter) that rotates at high speed (by centrifugal action). The film of liquid that is formed moves outwards over the surface of the vessel to the residue-collection pipe. The vessel is heated by radiant heaters. Condensers and a collection device are located close to the inner surface of the rotor. Therefore condensate is collected into other vessels while the residue is collected at bottom of the vessel which is recirculated through a feed pipe for further distillation.
Uses:
- For separation of vitamins (example Vitamin A and E)
- For purification of chemicals
- For purification of oils
Make sure you also check our other amazing Article on : Steam Distillation