Nomenclature of Optical Isomers: The d/l system was developed by Fischer and Rosanoff in around 1900. Arbitrarily, (+) glyceraldehyde was defined as being D because the OH group attached to the C2 is on the right-hand side of the molecule. While (–) glyceraldehyde was defined as L because the OH group is on the left-hand side.
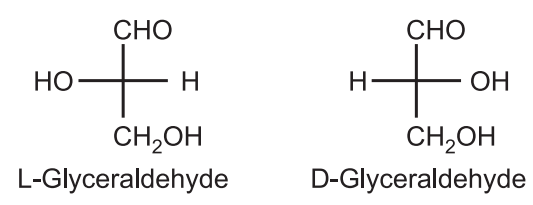
(1) The d/l system (named after Latin dexter and leaves, right and left) names the molecule by relating them to the molecule glyceraldehyde. This system of nomenclature represents an older system for distinguishing enantiomers of amino acids and carbohydrates. This arbitrary type of configuration (d/l system) is known as Relative Configuration.
(a) To name complex amino acids and carbohydrates in Fischer projection, take the carbonyl group (aldehyde, ketone, or carboxylic acid) on the top and CH2OH on the bottom.
(b) The D descriptor is used when the –OH or –NH2 on the 2nd carbon (from bottom) points to the right and L is used when the –OH or –NH2 points to the left. Thus, from the stereochemistry of only one stereocenter (i.e. 2nd carbon from bottom) the stereochemistry of all other stereocenters in the molecule is defined.
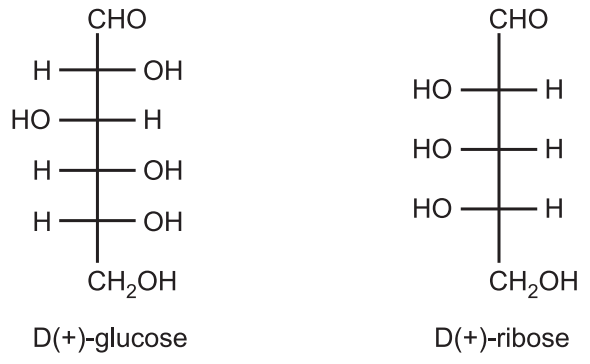
(c) The d/l nomenclature does not indicate which enantiomer is dextrorotatory and which is levorotatory. It just says that the compound’s stereochemistry is related to that of dextro – or Levo – enantiomer of glyceraldehyde. For example, d-fructose is levorotatory. Hence, it is stated that all-natural amino acids are L while natural carbohydrates are D. Thus, (+) glucose has the D-configuration, and (+) ribose has the L-configuration.
The Cahn Ingold Prelog (CIP) Sequence Rule
An absolute configuration refers to the spatial arrangement of the atoms of the chiral molecules and its stereochemical description using terms (R) or (S). Cahn, Ingold, and Prelog introduce Sequence Rules to assign an order of priority to the atoms or the groups directly attached to a stereocenter. The absolute configuration of a given stereocenter is defined as either (R) or (S) by applying these rules.
Rule 1: Atom of higher atomic number is given priority over those of lower atomic number
e.g., I > Br > Cl > F >O > N > C > H.
Rule 2: Isotope of higher atomic weight takes precedence.
e.g., 3H (tritium) > 2H (deuterium) > 1H (hydrogen)
Rule 3: When two or more atoms directly attached to a stereocenter are the same, the order of priority depends on the next atom along the chain.
e.g., –CO2CH3 > –CO2H > CONH2 > COCH3 > CHO > CH2OH
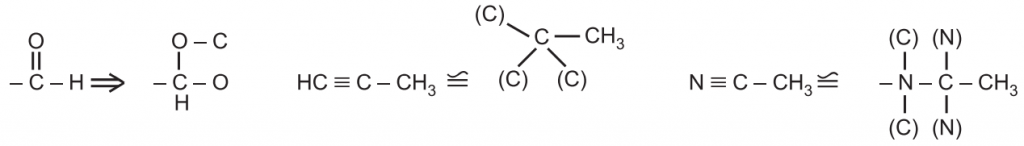
Rule 4: If an atom is double bonded to another atom, treat it as if it were singly bonded to two of those atoms. If an atom is triply bonded to another atom, treat it as if it were singly bonded to three of these atoms. Convert the unsaturated group directly attached to the stereocenter into the saturated group to decide the order of priority e.g.,
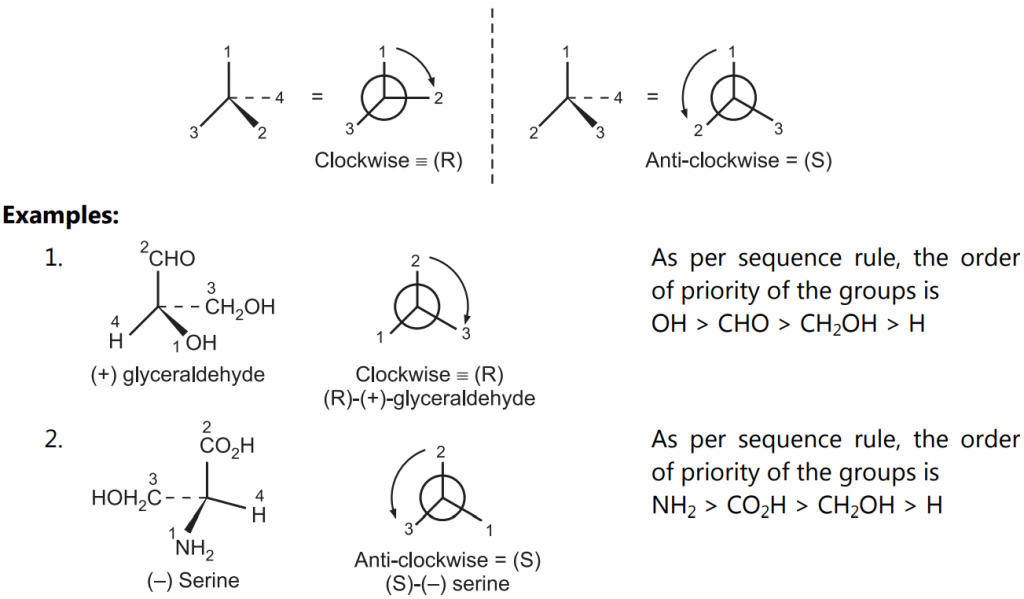
Applying the above sequence rules, assign the numbers to the functional groups as per the order of priority. Draw a generic tetrahedral center and view the molecule so that the atom/group with the lowest priority should project maximum away in space. A clockwise decreasing order is assigned the (R) – configuration while an anti-clockwise decreasing order is assigned the (S)-configuration.
Rule 5: A longer group may not necessarily have a higher priority over another smaller group.
e.g. –CH2Cl has a higher priority than –CH2CH2CH2CH3.
Rule 6: If the lowest priority group is in the front of the plane of the molecule then the assignment is reversed. i.e., clockwise is S and anticlockwise is R.
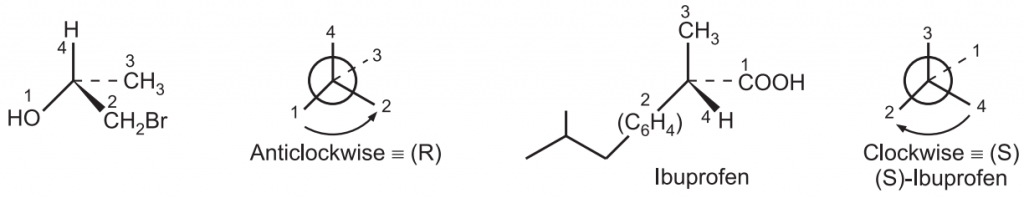
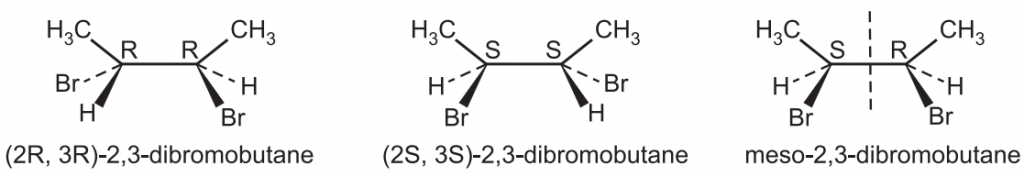
Rule 7: If there are multiple chiral carbons in a molecule, the configuration of the entire molecule can be defined by using a number that specifies the location of the stereocenter preceding configuration e.g., (1R, 4S). e.g.,
Make sure you also check our other amazing Article on : Stereoisomerism