Ocular Drug Delivery System: The eye is a complex organ with unique anatomy and physiology. The structure of the eye can be divided into two main parts: the anterior segment and the posterior segment. The anterior segment of the eye occupies approximately one-third while the remaining portion is occupied by the posterior segment. Tissues such as the cornea, conjunctiva, aqueous humor, iris, ciliary body, and lens make up the anterior portion. Back of the eye or posterior segment of the eye include; sclera, choroid, retinal pigment epithelium, neural retina, optic nerve, and vitreous humor. The anterior and posterior segment of the eye is affected by various vision-threatening diseases. Diseases affecting the anterior segment include, but are not limited to glaucoma, allergic conjunctivitis, anterior uveitis, and cataract. Age-related macular degeneration (AMD) and diabetic retinopathy are the most prevalent diseases affecting the posterior segment of the eye.
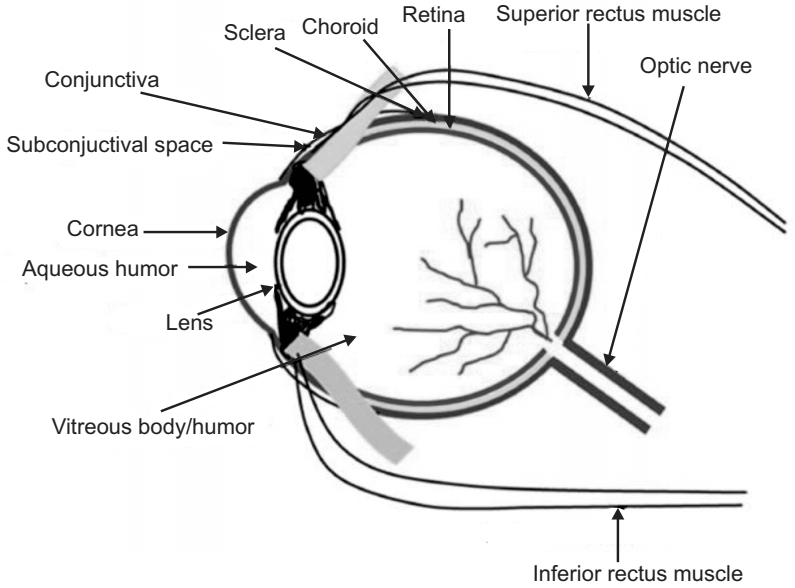
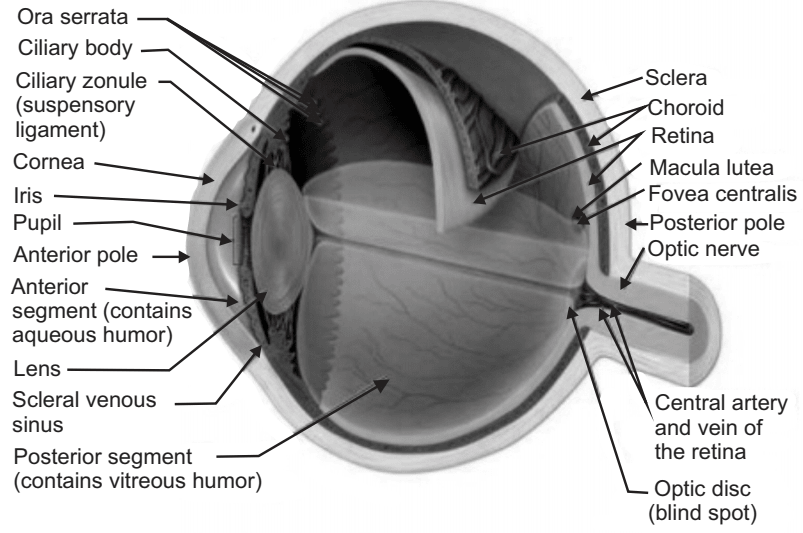
Ocular administration of the drug is primarily associated with the need to treat Ophthalmic diseases. The eye is the site for topical administration of medication. The main objective for the ophthalmic drug delivery is that it should sustain the drug release and remain in the vicinity of the front of the eye for a prolonged period.
There are specialized dosage forms designed to be instilled onto the external surface of the eye (topical), administered inside (intraocular) or adjacent (periocular) to the eye, or used in conjunction with an ophthalmic device.
The novel approach of drug delivery in which the drug is instilled on the cul de sac cavity of the eye (space between eyelids and eyeballs) is known as Ocular Drug Delivery System.
The most commonly used employed dosage forms are the Solutions, Suspensions, and Ointments. The newest dosage forms are the Gels, Gel forming Solutions, Ocular inserts, intravitreal injections, and implants.
Composition of Eye
Table of Contents
Eye is composed of Water – 98%, Solid – 1.8%, Protein – 0.67%, Sugar – 0.65%, NaCl – 0.66%, other mineral element Sodium, Potassium and Ammonia – 0.79%.
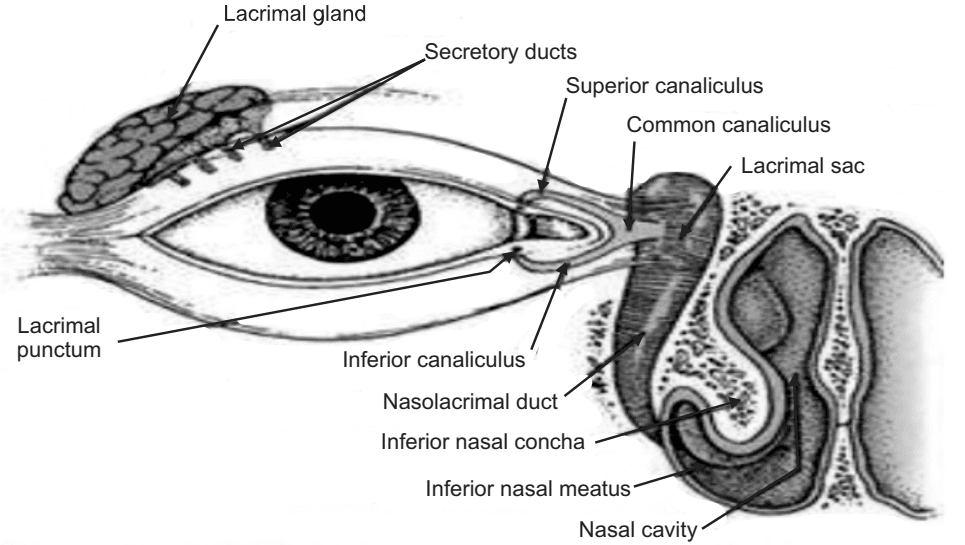
Mechanism of Ocular Absorption
There are two types:
1. Non-Corneal Absorption: The penetration is across Sclera and Conjunctiva into intraocular tissues. It is non-productive as it restrains entry of the drug into aqueous Humour.
2. Corneal Absorption: The outer epithelium with a rate-limiting barrier, with pore size 60A and allowing only small ionic and lipophilic molecules. The trans-cellular transport is between corneal epithelium and stroma. Drugs absorbed through cornea discharge through aqueous humor into systemic routes.
Factors Affecting Intraocular Bioavailability
- Lacrimal fluids.
- Naso-lacrimal drainage.
- Interaction of drug with proteins of lacrimal fluids.
- Dilution with tears.
- Cornear barriers.
- Active ion transport at the cornea.
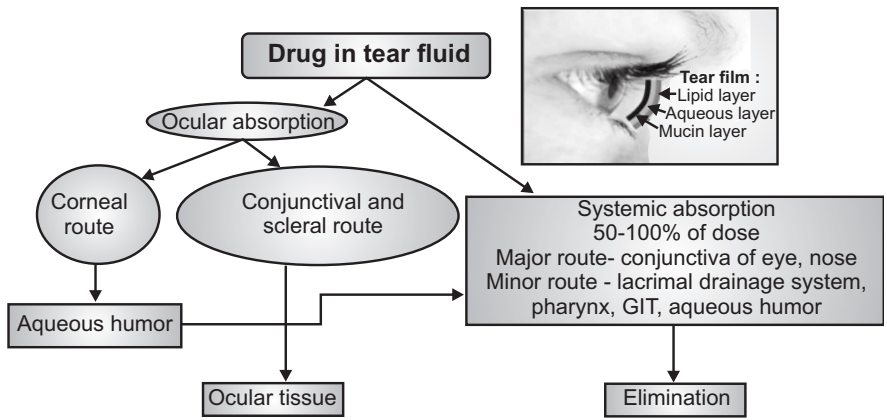
Ophthalmic dosage forms are sterile products essentially free from foreign particles, suitably compounded, and packaged for instillation into the eye.
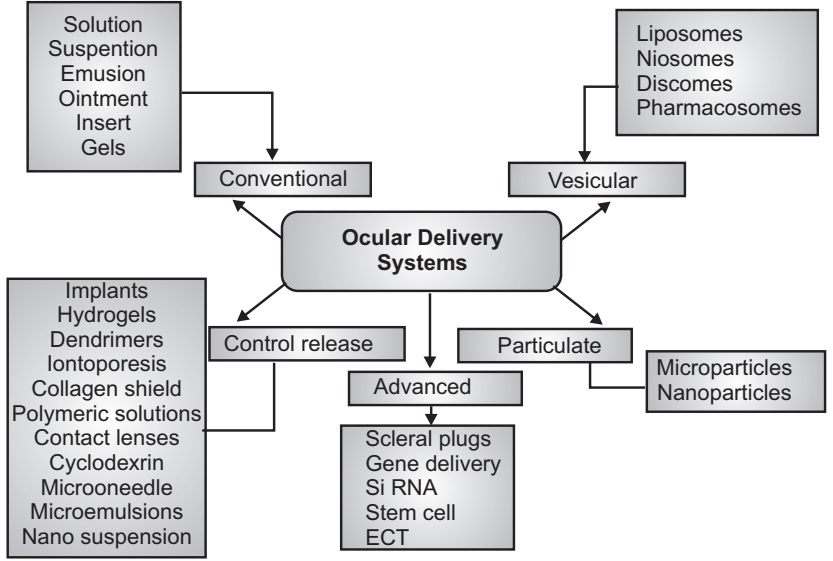
Some of the dosage forms have been developed for Ocular drug delivery systems.
Advantages of Ocular Drug Delivery System
- It can be easily administered.
- They have quick absorption and effect.
- Less visual and systemic side effects.
- Better patient compliance.
Disadvantages of Ocular Drug Delivery System
- The residence time of the drug at the eye surface is less.
- Poor bioavailability.
- The instability of the dissolved drug.
- The low concentration of preservatives reduces shelf life after opening the bottle.
Ideal Characteristics of Ocular Drug Delivery System
- It should be sterile.
- It should be isotonic to body fluids.
- Buffer/pH adjustment.
- Less drainage tendency.
- Minimum protein binding.
Barriers to Ocular Drug Delivery System
The reason why it is difficult to achieve relevant therapeutic doses within the eye is primarily due to the presence of multiple barriers. When a dosage form is either administered topically or systemically, it faces multiple obstacles before it reaches its site of action. As a result, ocular bioavailability from topically administered drugs is usually only 1%-7% of the applied dose. These barriers can be broadly classified as anatomical barriers and physiological barriers.
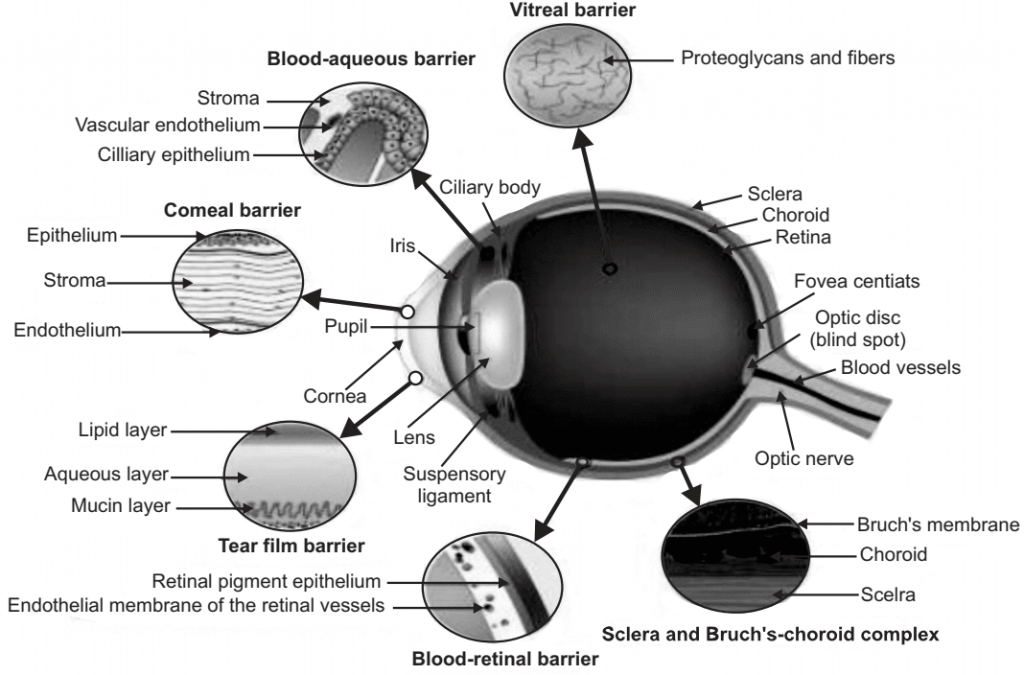
1. Anatomical Barriers:
When a dosage form is topically administered, there are two routes of entry: either through the cornea or via the non-corneal route.
(a) The cornea is a very tight multilayered tissue that is mainly composed of five sections:
- Epithelium-Principle barrier (Hydrophilic drug transport through intercellular spaces).
- Bowman’s membrane.
- Stroma-multiple layers of collagen fibers containing pores and channels (for lipophilic drug, significant barrier).
- Descemet’s membrane.
- Endothelium.
Prevention: Optimum bioavailability, the right balance of lipophilic and hydrophilicity.
(b) Non-corneal route bypasses the cornea and involves movement across conjunctiva and sclera.
(c) Conjunctiva: It is more permeable than cornea for hydrophilic molecules.
2. Physiological Barriers:
The eye’s primary line of defense is its tear film. The bioavailability of topically administered drugs is further reduced by precorneal factors such as; solution drainage, tear dilution, tear turnover, and increased lacrimation. This in turn lowers the exact time for absorption leading to reduced bioavailability. Prevention: So, the drugs administered as eye drops need to be isotonic and nonirritating to prevent significant precorneal loss.
3. Blood Ocular Barrier:
This barrier normally keeps most drugs out of the eye, but inflammation breaks down this barrier allowing drugs and large molecules to penetrate the eye.
(a) Blood aqueous barrier: The ciliary epithelium and capillaries of the iris.
(b) Blood retinal barrier: Non-fenestrated capillaries of the retinal circulation and tight junctions between retinal epithelial cells preventing passage of large molecules from choriocapillaris into the retina.
4. Drug and Dosage Form Related Factors:
(a) Solubility: Solubility is dependent on the pKa of the drug and the pH of the solution.
(b) Lipophilicity: Lipophilicity and corneal permeability display a sigmoidal relationship. This is because of the differential permeability of the different layers of cornea towards lipophilic drugs. Lipophilic drugs tend to permeate easily through the epithelial layers of the cornea and the hydrophilicity of the inner layer of the cornea (stroma) requires higher hydrophilicity for optimal permeation.
(c) Molecular weight and size: The weight and size of molecules play a critical role in deciding their overall permeability through the paracellular route. Molecules having a molecular weight less than 500 Dalton can permeate readily.
- The conjunctiva has a larger paracellular pore diameter thus allowing permeation of larger molecules such as small and medium-size peptides (5000-10000 Daltons).
- Permeation across the sclera occurs through the aqueous pores and the molecular size of the solute can be the determining factor. Sucrose (molecular weight – 342 Daltons) permeates 16 times faster than inulin (molecular weight – 5000 Daltons). Scleral permeability is approximately half of the conjunctiva but much higher than the cornea.
Methods to Overcome Barriers to Ocular Drug Delivery
Drug delivery through topical or systemic routes faces several challenges limiting their success. Advancements in drug design, drug formulation, and devices have led to successful products. But the scientists have experimented with alternate routes of drug delivery that can overcome barriers presented by the more conventional routes. Injections through visible portions of the sclera targeting various sections of ocular structures are routinely carried out by a trained specialist.
1. Intravitreal Injections: Intravitreal injection (IVI) involves delivering the drug formulation directly into the vitreous humor through pars plana. This method provides direct access to the vitreous and avoids both the cornea and also the scleral blood vessels. Formulations such as; solution, suspension, or a depot formulation can be administered through this route. Drug elimination occurs either through the retina or the anterior chamber through the aqueous humor following a first-order rate of decline. This rate of elimination has a linear correlation with the molecular weight of the drug. Larger molecules tend to have longer half-lives as high as several weeks as compared to less than 3 days for low molecular weight compounds. IVI administration is associated with adverse effects such as; retinal detachment, cataract, hyperemia, and endophthalmitis. Sustained release drug delivery systems can help by lowering the frequency of administration and thus allow for better patient compliance.
2. Subconjunctival Injections: This injection delivers the drug beneath the conjunctival membrane that lines the inner surface of the eyelid. It allows for circumvention of both cornea and conjunctiva allowing the drug direct access to the sclera. It is much less invasive with lesser side effects when compared to intravitreal injections. The method is an excellent route for delivering hydrophilic drugs as it bypasses their rate-limiting barriers allowing more drugs to enter into the vitreous. It is an excellent route for delivering both depot-forming formulations as well as for the delivery of macromolecular drugs such as Avastin (bevacizumab: a recombinant monoclonal antibody against VEGF) and insulin.
3. Retrobulbar and Peribulbar Route: Retrobulbar injection is given through eyelid and orbital fascia and it places the drug into retrobulbar space. This mode administers the drug to the back of the eyeball and is used to deliver drugs such as; antibiotics and corticosteroids. This route is especially applicable for the delivery of anesthetic agents as it causes minor or no change in IOP though in certain orbital diseases the reverse is also possible. Yet, it is a very delicate procedure as it may damage the optic nerve and thus requires proper expertise and equipment.
The Peribulbar route for drug delivery involves injections above and/or below the globe. It is also a viable route for the delivery of anesthesia, especially in cases of cataract surgery. It is a safer route compared to the retrobulbar route with a reduced risk of injury. Though it is a safer method, unlike retrobulbar injection multiple cases of elevated intraocular pressure after peribulbar injections have been reported.
4. Sub-Tenon Injections: Sub-tenon injections are administered into a cavity between the tenon’s capsule and sclera using a blunt cannula. Pre-operative deep sedation is also not a requirement for this procedure. The sub-tenon route appears to be a better and safer route for delivering anesthesia relative to retrobulbar and peribulbar administration since it does not require sharp needles. Steroids injected through this route have also been shown to be effective in the treatment of uveitis, cystoid macular edema, complicating uveitis, and non-necrotizing scleritis.
5. Intracameral Injections: Intracameral route is similar to intravitreal injections, but this injection delivers the drug to the anterior chamber. Drugs administered through this route are limited to an anterior chamber with very limited access to the posterior segment. It is generally employed for anterior segment procedures such as cataract surgery. Clinical studies have reported that intracamerally delivered dexamethasone is effective in reducing postoperative inflammation in glaucomatous and non-glaucomatous patients. It is an efficient and often more cost-effective method of delivering antibiotics relative to topical antibiotics and antifungal agents.
Formulations for Ocular Drug Delivery System
1. Conventional Delivery Systems
- Eye drops
- Ointments and Gels
- Ocuserts and Lacriserts
2. Vesicular Systems
- Liposomes
- Niosomes and Discomes (Giant Niosomes)
3. Controlled Delivery Systems
- Implants
- Iontophoresis
- Dentrimer
- Microemulsion
- Nanosuspension
- Microneedle
- Mucoadhesive Polymers
Inserts
Classification of Inserts
- Non-Erodible Inserts: (a) Ocusert (b) Contact Lens
- Erodible Inserts: (a) Lacriserts (b) SODI (c) Mindisc
1. Non-Erodible Inserts:
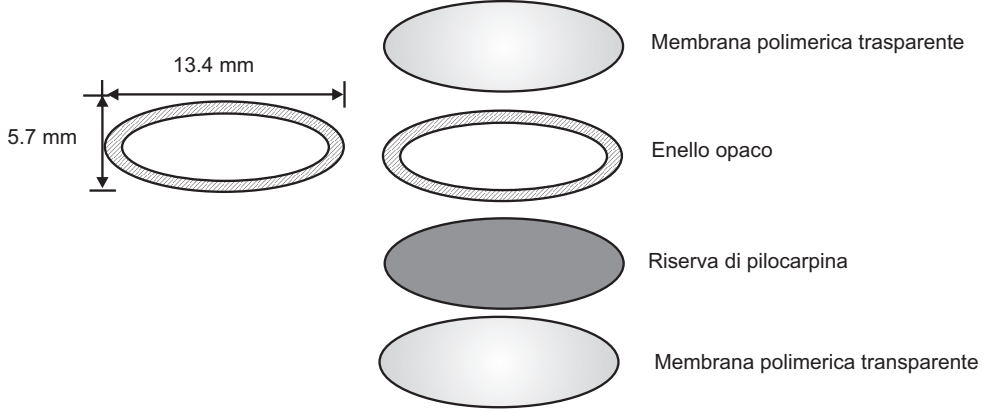
(a) Ocuserts: It is a flat, flexible, elliptical device designed to be placed in the inferior cul-de-sac between the sclera and eyelid which releases Pilocarpine continuously at a steady state for 7 days. It comprises 3 layers:
- Outer Layer: Ethylene-vinyl acetate copolymer layer.
- Inner Layer: Pilocarpine gelled with alginate main polymer.
- A retaining ring of EVA impregnated with titanium dioxide. Example: Pilo 20 (20 mg/hr), Pilo 40 (40 mg/hr).

(b) Contact Lens: These are circular-shaped structures; dyes may be added during the polymerization. Drug incorporation depends on whether the structure is Hydrophilic or Hydrophobic.
2. Erodible Inserts:
(a) Lacriserts: It is a sterile rod-shaped device composed of HPC without preservatives. Its weight is 5 mg, its diameter is 12.5 mm and length is 3.5 mm. It is used in dry eye treatment, Keratitis Sica.

(b) SODI (Soluble Ocular Drug Insert): Inserted in Inferior Fornix. It is a small water-soluble wafer-like insert, composed of acrylamide vinyl Pyrrolidone, Ethyl acrylate. It weighs 15-16 mg. It softens in 10-15 sec., turns in viscous liquids in 10-15 minutes, and after 30-60 minutes becomes the polymeric solution. It is used in the treatment of Glaucoma and Trachoma.
(c) Minidisc: It is made up of a counter disc with a convex front and concave back surface in contact with the eyeball. It measures 4-5 mm in diameter. It is composed of silicon-based prepolymer. E.g. Pilocarpine, Chloramphenicol.
Make sure you also check our other amazing Article on : Targeted Drug Delivery System
