Perchloric Acid in Glacial Acetic Acid (0.1 M)
Molecular formula: HClO4
Molecular weight: 100.46
Preparation: Mix 8.5 mL of perchloric acid with 500 mL of anhydrous glacial acetic acid and 25 mL of acetic anhydride, cool and add anhydrous glacial acetic acid to produce 1000 mL. Allow the prepared solution to stand for 1 day, for the excess acetic anhydride to be combined and carry out the determination of water. (Water content should be between 0.02 %- 0.05%).
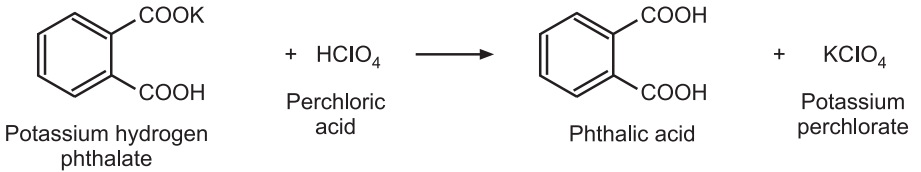
Standardization: Weigh accurately about 0.35 g of potassium hydrogen phthalate, previously powdered and dried at 120°C for 2 hours and dissolve it in 50 mL of anhydrous glacial acetic acid. Add 0.1 mL of crystal violet solution and titrate with the perchloric acid solution until the violet color changes to emerald green. Perform a blank determination and make any necessary corrections.
Reaction:
Factor Calculation:
1 mole of HClO4 ≅ 1 mole of potassium hydrogen phthalate
1000 mL of 1M HClO4 ≅ 204.2 g of C8H5KO4
1 mL of 0.1M HClO4 ≅ 0.02042 g of C8H5KO4
Make sure you also check our other amazing Article on: How do you make 0.5 M of sulphuric acid?