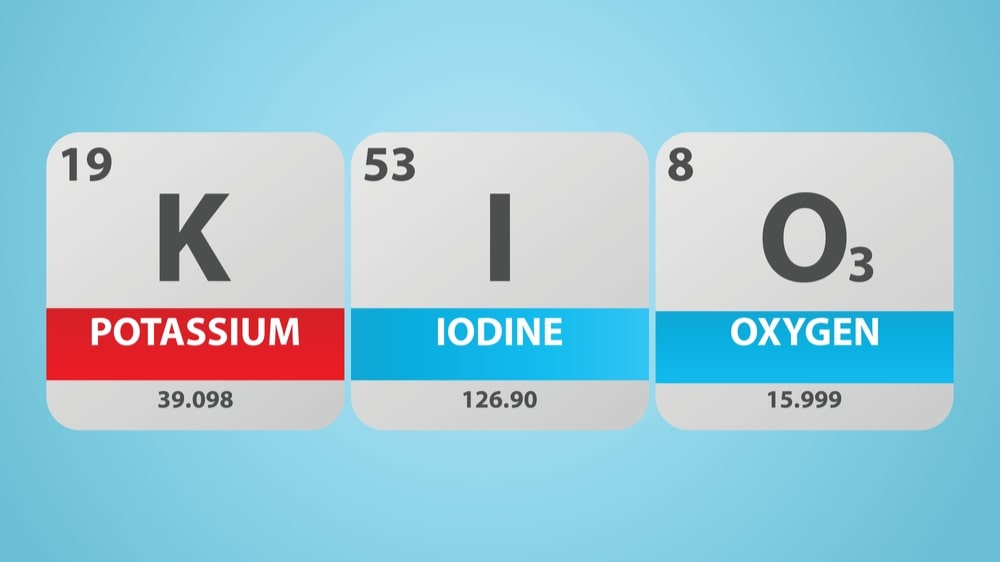
Potassium Iodate (0.05M)
Molecular formula: KIO3
Molecular weight: 214
Preparation: Weigh accurately 10.7 g of potassium iodate (pure standard) previously dried at 110°C to constant weight, transfer in a volumetric flask, and add sufficient water to produce 1000 mL.
Standardization: Dilute 25 mL of potassium iodate solution to 100 mL with water. To 25 mL of this solution add 2 g of potassium iodide and 1M H2SO4 and titrate with 0.05M sodium thiosulphate using starch solution as an indicator added towards the endpoint.
Reaction:
KIO3 + 5KI + 3H2SO4 → 3I2 + 3K2SO4 + 3H2O
3[2Na2S2O3 + I2 → Na2S4O6 + 2NaI]
Factor calculation:
Since,
6Na2S2O3 ≅ 3I2 ≅ potassium iodate (KIO3)
6 moles of Na2S2O3 ≅ 1 mole of KIO3
6000 mL of 1M Na2S2O3 ≅ 214 g of KIO3
1 mL of 0.05M Na2S2O3 ≅ 0.00178 g of KIO3
Make sure you also check our other amazing Article on: What is 0.1 M tetrabutylammonium hydroxide solution?