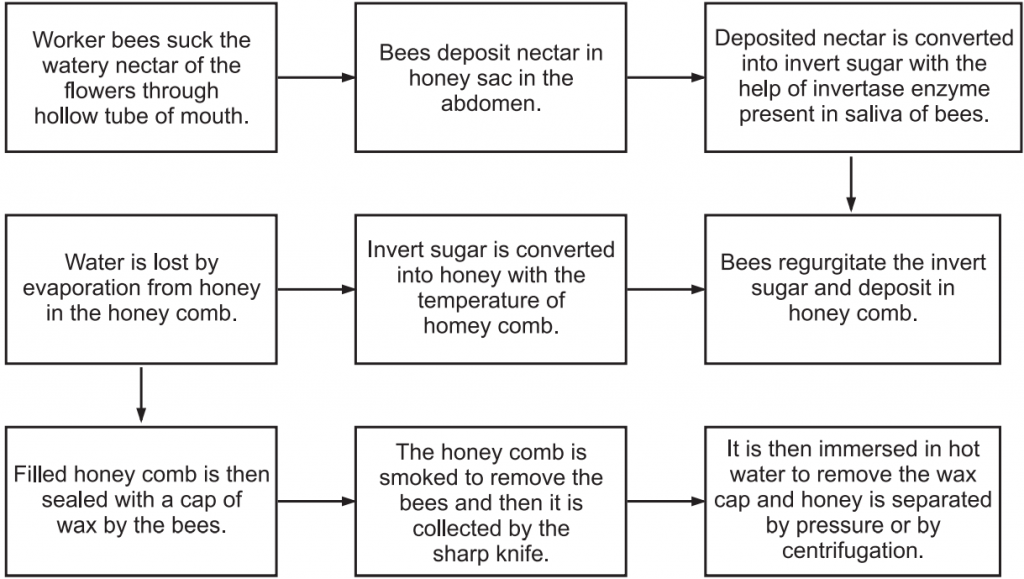
Preparation of Honey: Honey is a saccharine liquid prepared from the nectar of the flower by the honey bees that are deposited in the honeycomb. The bees are Apis mellifera, Apis dorsata, and others.
Family: Apidae.
Geographical Location: Honey is produced in many parts of the world like Africa, Australia, Newzealand, Asia. In India, it is abundantly produced in the forest area of all the states. Maximum honey production is obtained from the Himalayan forest region, Karnataka, West Bengal, Madhya Pradesh and Andhra Pradesh.
Preparation of Honey: It is described in Fig.1.
Chemical Constituents:
Table of Contents
Honey is essentially a concentrated aqueous solution of inverted sugar and contains a highly complex mixture of other carbohydrates, a variety of enzymes, amino acids, organic acids, minerals, vitamins, aromatic substances, pigments, waxes, etc. The main sugars present in honey are fructose (38%) and glucose (31%). The saccharose content varies by the state of maturity of the honey, and the composition of the oligosaccharide fraction is determined by the plants used in the production process. Honey contains free amino acids in quantities of around 0.1% of the dry product. Proline is the major amino acid, but other amino acids like arginine, alanine, glutamic acid, aspartic acid, lysine, glycine, and leucine are also present. The main acid in honey is gluconic acid and smaller quantities are also found of lactic, citric, succinic, formic, malic, acetic, malic and oxalic acids.
Properties:
The physical properties of honey vary, depending on water content, the type of flora used to produce it, temperature, and the proportion of the specific sugars it contains.
- Honey is translucent, white to pale yellow liquid.
- Odour is pleasant and characteristic whereas taste is sweet.
- It is soluble in water but insoluble in alcohol.
- Fresh honey is a supersaturated liquid, containing more sugar than water.
- It is hygroscopic.
- At room temperature, honey is a supercooled liquid, in which the glucose will precipitate into solid granules.
- The melting point of crystallized honey is between 40 and 50°C.
- Honey has a glass transition between −42 and −51°C.
- Honey contains 16% humidity, at 70°C.
- Honey has more viscosity than water and is around 10,000 cps (counts per second).
- The refractive index for honey ranges from 1.504 at 13% humidity to 1.474 at 25%.
- Honey also contains acids, which act as catalysts.
- The average pH of honey is 3.9, but it can range from 3.4 to 6.1.
- A product that contains crystallized dextrose is called granulated honey.
Uses: The medicinal uses of honey are as wound dressing material, antimicrobial, anti-inflammatory, antiseptic and reduce tissue damages. In Ayurveda, honey is used for both internal and external applications. It is mainly used for the treatment of eye diseases, cough, thirst, leprosy, diabetes, obesity, worm infestation, vomiting, asthma and diarrhoea.
According to Ayurveda, there are eight different types of honey:
- Makshikam: It is used in the treatment of eye disease, hepatitis, piles, asthma, cough etc.
- Bhraamaram: It is used in the treatment of vomiting with blood.
- Kshoudram: It is used in the treatment of diabetes.
- Pauthikam: It is used in the treatment of diabetes and urinary infection.
- Chathram: It is used in the treatment of worm infestation.
- Aardhyam: It is effective for eye diseases, cough and anaemic conditions.
- Ouddalakam: It is used in the treatment of leprosy and poisoning conditions.
- Daalam: It increases digestion and helps in the treatment of cough and vomiting.
Chemical Tests:
1. The amount of hydroxymethylfurfural (HMF) in honey can be determined by Reflectoquant® HMF test by dilute weighed honey sample with distilled water (1 : 4) and by putting the test strip into reflectometer.
2. Sample honey is mixed with ether and then evaporated at room temperature. Then dilute HCl and resorcinol are added to the solution. The acid layer will not form any red or pink colour.
Adulteration:
Gas Chromatography (GC), Liquid Chromatography (LC) analysis, Near-Infrared Transflectance Spectroscopy (NIR) etc. are suitable for use as a screening technique in the quality control of honey. Generally, corn syrup, flour, molasses, glucose, starch, dextrose and other similar products are used as an adulterant for honey.
(a) Honey with Sugar Solution (Sugar + Water): To detect the adulterant, (a) Pure honey is always in a semi-solid state. (b) If adulterated honey is poured into water it will dissolve immediately. If honey is pure, it will not dissolve so soon.
(b) Honey with Cane Sugar: Microscopic analysis. Cane sugar exhibited parenchyma cells, single ring vessels and epidermal cells, whereas all these characters are absent in honey.
(c) Honey with Invert Sugar: It can be checked with the help of Fisher’s test. A sample of honey is mixed with ether and then evaporated at room temperature. Then dilute HCl and resorcinol are added to the solution. An acid layer will not form any red or pink colour.
(d) Honey with Glucose: This can be identified by the iodine test. Sample honey is mixed with the same quantity of water and then potassium iodide is added. The solution becomes red or violet. This indicates the presence of glucose. This test is negative for pure honey.
(e) Honey with Commercial Sugar: This can be identified by the Aniline chloride test. Sample honey + mixture of hydrochloric acid and Aniline (3 : 1) → crimson red colour or the orange colour forms due to formation of aniline chloride by commercial sugar. This test is negative for pure honey.
(f) Honey with Starch or Flour: Starch or flour is added to honey for a simple reason, to increase its weight and whiteness. One can add cold water to sample honey and thus be sure whether it is free from flour and starch or not. If they are present in honey then honey falls to the bottom of the vessel. When they are exposed to heat, they remain in the liquid form, but upon cooling down they turn hard.
Make sure you also check our other amazing Article on : Agar
