Racemic Modification: A racemic modification or racemate is a 1:1 mixture of (+) and (–) enantiomers. When enantiomers are mixed in equal amounts, the rotation caused by a molecule of one isomer is exactly canceled by an equal and opposite rotation caused by a molecule of its enantiomer. Hence, the overall optical rotation of the racemate is zero. A racemic modification is thus optically inactive. The prefix (±) is used to denote the racemic nature of the sample. e.g., (±)-2-methyl-1-butanol.
When one of the starting materials is chiral, the product of the reaction will always be formed as a racemate in the absence of a chiral catalyst.
However, biologically active pure enantiomers can be synthesized in the presence of chiral catalysts or agents.
Methods of Racemic Modification
(a) Mixing: A racemic modification may be achieved by intimate mixing of exactly equal amounts of Dextro (+) and Levo (–) isomers.
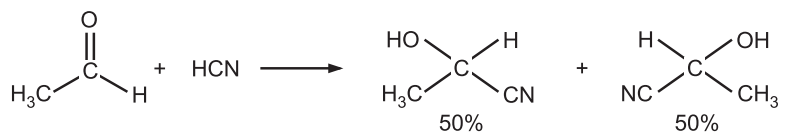
(b) Chemical synthesis: When one of the starting materials is chiral the product of the reaction will always be formed as a racemate in the absence of a chiral catalyst. e.g., when hydrogen cyanide reacts with acetaldehyde (chiral), an equal number of mole of two enantiomeric forms of acetonitrile, CH3CHOHCN results.
(c) Thermal racemization: Racemization may occur when an optically active material is heated. It leads to temporarily breaking one of the 4 bonds to a stereocenter. The atom/group separated exchanges the position and joins back to stereocenter to form another enantiomer e.g., the distillation of optically active enantiomer of α-phenethyl chloride leads to its racemization.
(d) Walden inversion: The racemization of 2-isooctane by potassium iodide in refluxing acetone involves a process known as Walden inversion.
(e) Epimerization: It is the change in the configuration at one asymmetric carbon atom in a compound having more than one stereocenters. It thus leads to the interconversion of diastereomers.
(f) Mutarotation: It is a spontaneous change with time, in the rotation of freshly prepared solutions of optically active substance till it reaches an equilibrium. Mutarotation is the result of either epimerization or a spontaneous structural change. The rate of mutarotation depends on temperature, solvent, and catalyst. For example, the mutarotation of glucose is known to be acid-base catalyzed.
Resolution of Racemic Mixture
The process of separating a racemate into pure enantiomers is known as resolution. Enantiomers have identical physical properties (b.p., m.p., solubility) and hence it is difficult to separate enantiomers using conventional methods. If a pair of enantiomers is converted into a pair of diastereomers, the diastereomers can be separated easily utilizing the difference in their physical properties. Once separated, the pure enantiomer may be regenerated back from its respective diastereomer.
For example,
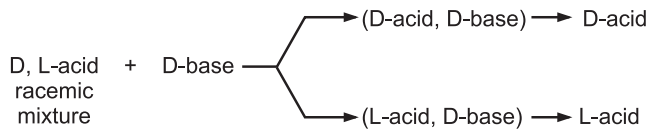
(i) A racemic mixture of enantiomers of an acid can be converted to a salt using a chiral base having D-configuration. The salt obtained contains a mixture of two diastereomers: (D acid, D base) and (L acid, D base). Due to differences in their physical properties, the diastereomeric salts are fully separated. Dissociation of separated diastereomeric salt leads to regeneration of D-acid and L-acid respectively.
Racemic acids may be resolved using commercially available chiral bases like brucine, strychnine, l-phenyl ethanamine. Similarly, racemic bases may be resolved using chiral acids such as (+) tartaric acid, (–) malic acid, (–) mandelic acid, and (+) camphoric acid.
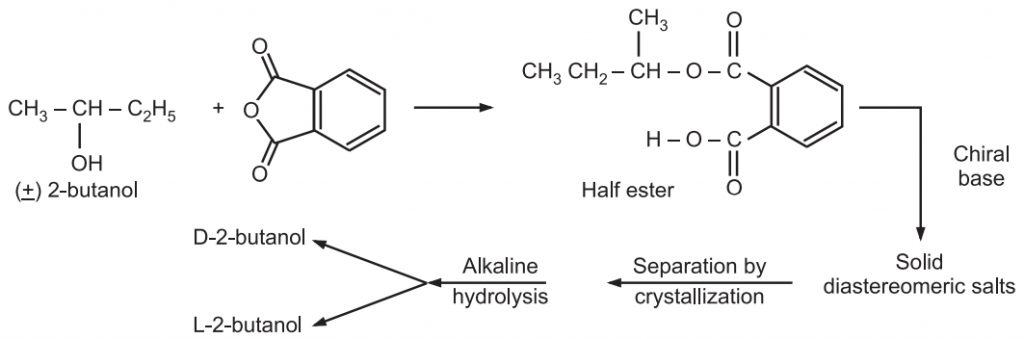
Racemic alcohol may be resolved by converting the racemate into a mixture of diastereomeric esters using a chiral acid. The separation of these diastereomeric esters becomes difficult if they are liquid. In such cases, instead of full ester, the half ester is synthesized containing one free carboxylic group. A chiral base, brucine then forms solid diastereomeric salts which can be later separated by crystallization. The pure enantiomer of 2-butanol is regenerated through hydrolysis of respective diastereomeric salt.
(ii) Resolution by biochemical means: Certain mold, bacteria or fungi selectively destroy one enantiomer at a faster rate than the other enantiomer. For example, the mold Penicillium glaucum if allowed to grow with the racemic mixture, it selectively destroys the dextro isomer leaving pure Levo isomer behind.
Advantages of Resolution:
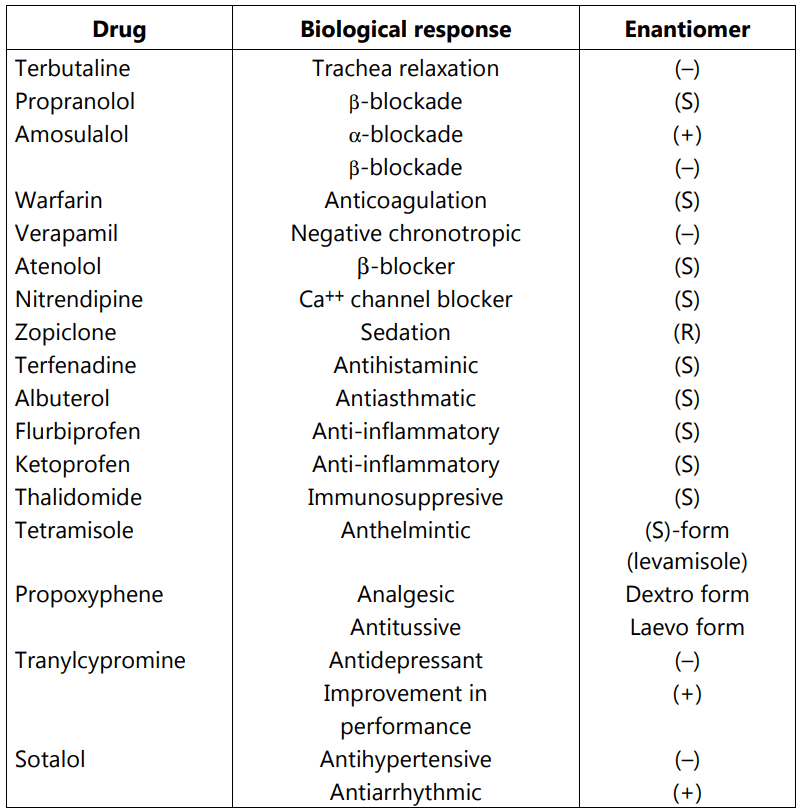
(i) To avoid side effects of unwanted enantiomer leading to improved therapeutic profile and fewer chances of a drug interaction.
(ii) Reduction in the therapeutic dose and hence the cost of treatment.
(iii) Lesser metabolic, renal and hepatic load of a drug on the body as the dose (for a pure enantiomer) reduces to half of the racemic mixture.
Make sure you also check our other amazing Article on : Elements of Symmetry