Resins are amorphous mixtures of essential oils and oxygenated products of terpenes, transparent or translucent solids, semi-solid or liquid substances. They have complex chemical nature and contain a large no of carbon atoms. It is insoluble in water and heavier than water but they are soluble in non-polar solvents like benzene or ether, volatile oils, fixed oils and alcohol. It is hard, non-conductor of electricity and combustible. They soften and finally melt upon heating. They are usually formed in schizogenous glands, lysigenous glands, or ducts as the end product of metabolism. The pharmaceutical applications of resins are a local irritant, local cathartic (e.g. Jalap, Ipomoea), as anticancer (podophyllum), in bronchial asthma (Cannabis), used externally as a mild antiseptic in the form of tinctures (Benzoin), ointment and plasters (Turpentine and Colophony) and used in the preparation of emulsion and sustained-release formulations.
Classification of Resins
Table of Contents
Depending upon the type of the constituents
- Acid resins examples Colophony contains abietic acid, Copaiba (copaivic and oxycopaivic acid), Myrrh (Commiphoric acid), etc.
- Ester resins examples Benzoin (Coniferyl benzoate), Storax (Cinnamyl cinnamate) etc.
- Resin alcohols examples Peru balsam (Peruresinotannol), Guaiacum resin (Guaic resinol).
Depending upon the combination with other Constituents
- Gum resin: Gum resins are in a homogenous combination of gum and resin. These are always associated with small quantities of other substances like bitter principle, enzymes, and volatile oils etc. It may consist of two or more glycosidal acids in various proportions and contains a trace amount of nitrogen e.g. Myrrh.
- Oleo resin: When resins are in homogenous combination with volatile oils or oily liquids, are called oleoresin. They are secreted in schizogenous or schizolysigenous ducts. Ginger, Capsicum, Turpentine oil.
- Oleo gum resin: These resins are in homogenous combination with volatile oil and gum. e.g. Asafoetida.
- Balsam resin: Those oleoresins which contain aromatic acids like benzoic acid or Cinnamic acid are known as balsam resin e.g. Benzoin.
- Glycoresin: These are made up of resin along with sugars e.g. Jalap, Ipomoea.
Some resins are complex natural substances not having transpose any specific chemical property, are chemically inert and do not get hydrolysed are known as resenes. A few examples are asafoetida, colophony etc.
Properties of Resins
- These are amorphous and brittle.
- They occur in translucent hard solid form.
- The resin softens and finally melted upon heating.
- They have specific gravity ranges from 0.9 to 1.25.
- When burnt, they produce a smoky flame.
- They are bad conductors of electricity.
- They are soluble in organic solvents like alcohol, ether and chloroform.
- They are insoluble in water.
- The resin film formed upon drying becomes hard and transparent which is unaffected by moisture and air.
- The majority of resins undergo slow atmospheric oxidation which darkens their colour and impaired solubility.
Distribution of Resins
Resins are abundantly distributed in plants and rarely in insects (Example- Shellac). It is present in ducts or cavities which are called schizolysigenous ducts. 2 types of resin exist in nature.
- Normal or Physiological resin: These are performed in the plants and their yield increases upon injury or incision. Example- Resin of Pinus.
- Abnormal or Pathological resin: This type of resin is formed only upon injury or incision made to the plant. Example- Benzoin, Tolu Balsam.
Chemical Test of Resins
- Solubility test: Resin dissolves when treated with organic solvents like alcohol, ether or chloroform etc.
- Ignition test: They produces smoky flame upon burning.
- HCl test: The drug is treated with hydrochloric acid which forms pink colour, ensuring the presence of resins.
- Ferric chloride test: The greenish-blue colour develops when the drug is treated with ferric chloride solution. This indicates the presence of resins.
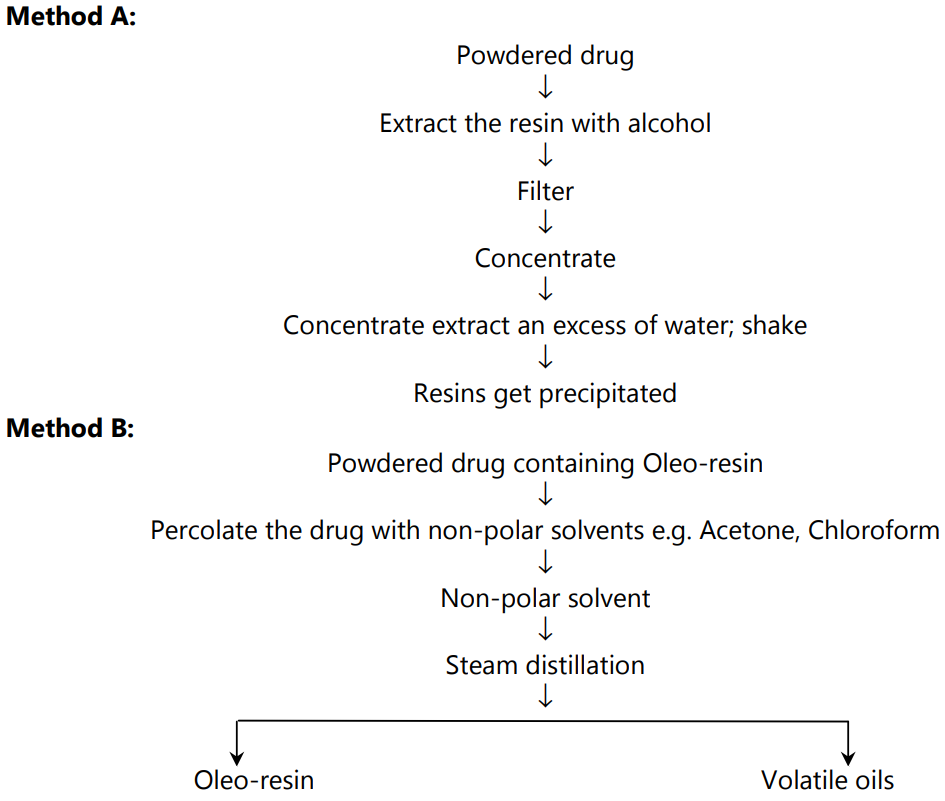
Extraction and Isolation of Resins
Make sure you also check our other amazing Article on : Pale Catechu
