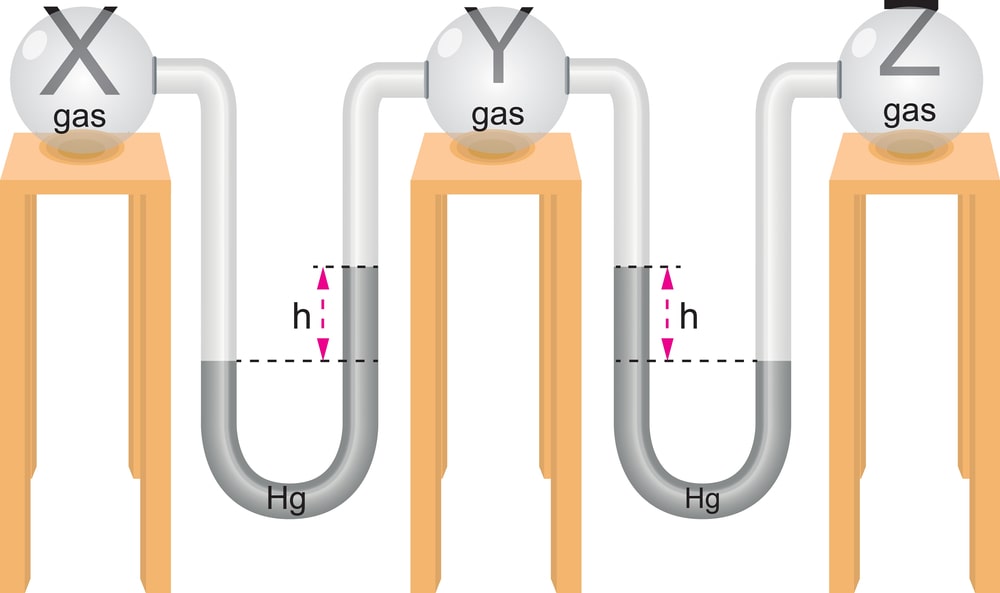
Simple U-tube Manometer: A manometer is a device to measure pressures. A common simple manometer consists of a U-shaped tube of glass filled with some liquid. In its simplest form, this type of manometer consists of an incompressible fluid like water or mercury. Typically it is mercury because of its high density.
Consider a U-shaped tube whose both ends are open to the atmosphere is filled with a liquid. The points A and B, Fig. 1(a), are at atmospheric pressure and the same vertical height. In another case, Fig. 1(b), consider that the left arm of the U-tube top end is closed and there is a sample of gas in the closed end of the tube. The right side of the tube remains open to the atmosphere. Point A, then, is at atmospheric pressure. Point C is at the pressure of the gas in the closed end of the tube. Point B has a pressure greater than atmospheric pressure due to the weight of the column of liquid of height h. Point C is at the same height as B, so it has the same pressure as point B. Thus, pressure at point C is equal to the pressure of the gas in the closed end of the tube. The pressure of the gas trapped in the closed end of the tube is greater than the atmospheric pressure by the amount of pressure exerted by the column of liquid of height h.
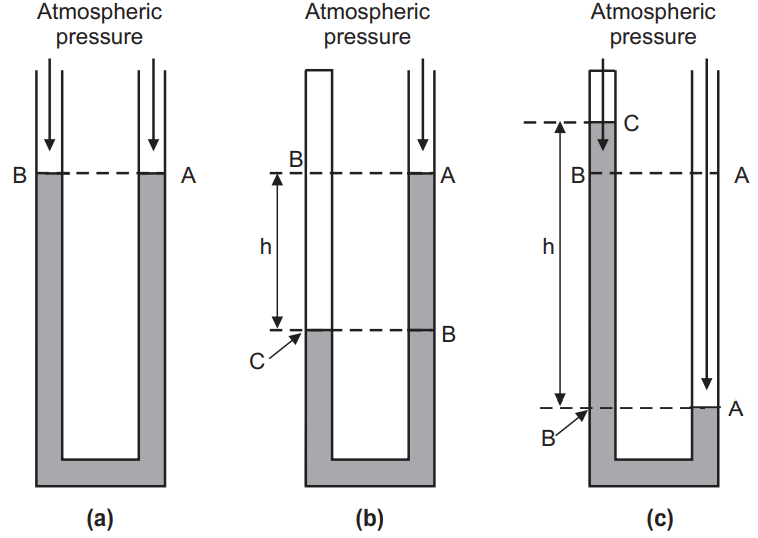
Another possible arrangement of the manometer where the top of the left side of the tube is closed and the closed end of the tube contains a sample of gas or it contains a vacuum, Fig. 1(c). Point A is at atmospheric pressure. Point C is at some pressure if it contains gas in the closed end of the tube. Since point B is at the same height as point A, it is at atmospheric pressure. But the pressure at point B is also the sum of the pressure at point C and the pressure exerted by the weight of the column of liquid of height h in the tube. Thus, it can be concluded that pressure at point C is less than atmospheric pressure by the amount of pressure exerted by the column of liquid of height h. If the closed end of the tube contains a vacuum, then the pressure at point C is zero, and atmospheric pressure is equal to the pressure exerted by the weight of the column of liquid of height h. The U-tube manometer is inexpensive and does not need calibration.
Pressure is defined as the force per area. The SI unit for pressure is the pascal, which is N/m2. Another common unit for measuring atmospheric pressure is mm of mercury, whose value is usually about 760 mm. If the closed end of the tube, Fig. 1(c), contains a vacuum, the height h is about 760 mm. In many situations, measuring pressures in units of length of the liquid in the manometer is perfectly adequate.
The pressure measurement in the manometer is calculated by considering a cylinder of liquid of height h and area A. The weight of the cylinder is its mass ‘m’ times the acceleration due to gravity ‘g’. This is the force exerted by the cylinder of liquid on whatever is just below it. It is expressed as:
F = m g …. (1)
The pressure ‘P’ is this force divided by the area ‘A’ of the face of the cylinder and is expressed as:
P =F/A ….(2)
The mass of the cylinder is the density of the liquid ‘ρ’ times the volume ‘V’.
m = ρ × V ….(3)
The volume is the area ‘A’ of the face of the cylinder times its height ‘h’.
V = A × h ….(4)
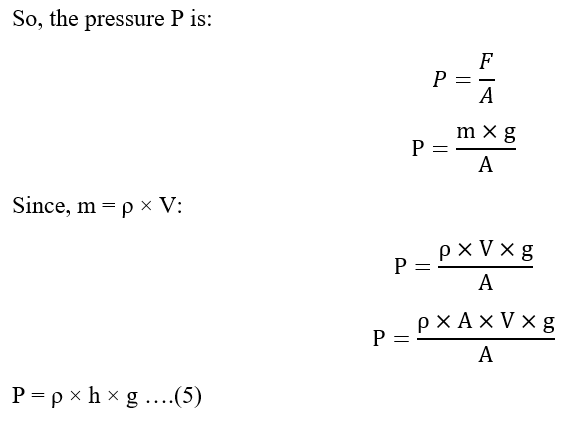
Make sure you also check our other amazing Article on : Working of Hammer mill