Sodium Hydroxide (1M)
Molecular formula: NaOH
Molecular weight: 40
Preparation: Dissolve 40 g of sodium hydroxide in sufficient carbon dioxide-free water to produce 1000 mL. Prepared solution stored in bottles with well-fitted suitable stoppers which prevent access to atmospheric carbon dioxide.
Standardization: Weigh accurately about 5 g of potassium hydrogen phthalate, previously powdered and dried at 120°C for 2 hours, and dissolve in 75 mL of carbon dioxide-free water. Add 0.1 mL of phenolphthalein solution and titrate with the sodium hydroxide solution until a permanent pink color is produced. Volumetric solutions of sodium hydroxide must be restandardise frequently.
Reaction:
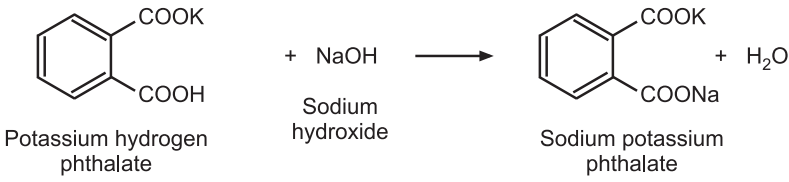
Factor Calculation:
1 mole of NaOH ≅ 1 mole of potassium hydrogen phthalate
1000 mL of 1M NaOH ≅ 204.2 g of C8H5KO4
1 mL of 1 M NaOH ≅ 0.2042 g of C8H5KO4
Make sure you also check our other amazing Article on: Different Techniques of Analysis
