Most of the raw materials for pharmaceutical products support microbial growth based on the nutritive properties and moisture contents. Microbiological contaminants are infectious materials, such as bacteria, mold, fungi, and viruses. These contaminants are often found in food and water and cause serious illnesses. Deterioration of pharmaceutical products by the contaminant microbes is known as microbial contamination. These microorganisms are cause spoilage of the product and results in loss of therapeutic properties. Various sources through which microbial contaminants gain entry are atmosphere, operators, raw materials from which products are manufactured, and equipments.
Atmosphere: Microbial growth in heating ventilation and air-conditioning (HVAC) systems and their contamination of the indoor air environment is a growing concern. A poor HVAC system is a potential source of growth of microbes and a transportation mode for dispersing contaminants throughout the manufacturing facility. The main reasons for contamination due to HVAC issues includes accumulations of organic material in or near HVAC air intakes, ineffective filtration of the supply air, the insufficient magnitude of pressure differentials causing the flow of reversal, erroneous ratio of fresh air to recirculated air, inability to access ventilation dampers and filters from outside the manufacturing areas and non-directional airflow within a production or primary packing areas.
Operators: The main reasons for the contamination from the operators include mainly lack of training, Direct contact between the operator’s hands and starting materials, primary packaging materials and intermediate or bulk product, Inadequate cleanliness, access of unauthorized operators into production, storage, and product control areas, malpractices like eating food, drinking beverages, or using tobacco in the storage and processing areas.
Raw materials: The raw materials used for production is a potential source of contamination. The main reasons for contamination from the raw materials include storage and handling mistakes causing mix-ups or selection errors, Contamination with microorganisms or other chemicals, degradation from exposure to excessive environmental conditions such as heat, cold, sunlight, moisture, etc., improper labeling, sampling, and testing.
Equipments: The equipment used in processing, holding, transferring, and packaging are the common source of pharmaceutical contamination. The main reasons for contamination include inappropriate design, size, material leading to corrosion and accumulation of static material, coolants, dirt, and sanitizing agents, improper cleaning and sanitization, design preventing proper cleaning and maintenance, improper calibration and irregular service, and, Deliberate use of defective equipment, etc.
Sources of Contaminants
- Air carrying dust: Dust is everywhere. Air is the carrier of dust particles. Very small dust particles that present inside the aseptic room are the source of contamination. Hence, a HEPA filter is used for the reduction of dust particles inside the room.
- The skin of the operators: Outer skin of the operators should smooth and covered properly. Any skin infections or rashes are the sources of microorganism growth inside the room.
- Movement of persons inside the aseptic room: The movement of persons inside the aseptic room should be restricted. A person walking inside the room liberates 5000 bacteria/minute.
- Operator’s health: Persons who are inside the aseptic room, should be healthy and free from any infections. A single sneeze will produce up to 1 million bacteria.
- Manufacturing process: The manufacturing process generates contaminants. The main reasons for contamination during the manufacturing process are lack of dedicated facilities to manufacture a single product, inappropriate cleaning in-between batches to minimize the number of product changeovers, use of an open manufacturing system exposing the product to the immediate room environment, inappropriate zoning, lack of cleaning status labeling on all equipment and materials used within the manufacturing facility, etc.
Types of Microbial Contaminant
Microbial contamination is broadly classified into direct contamination and cross-contamination (Flowchart.1).
(a) Direct contamination: Contamination occurred by microbial components and poorly maintained heating, ventilation, and air conditioning system.
(b) Cross-contamination: It is the process by which microbes are spread indirectly from one to another through improper and unsterilized equipment.

Direct contamination is classified as follows:
(a) Direct Physical contamination: Examples: Particles, fibers, metal parts, etc.
(b) Direct chemical contamination: Examples: Moisture, gases, vapors, etc.
(c) Direct biological contamination: Example: Microorganisms like bacteria, viruses, molds, fungi, etc.
Cross-contamination is classified as follows:
(a) Physical cross-contamination: Example: Leakage of oil seal from the reactor.
(b) Chemical cross-contamination: Examples: Moisture content is increased when a product is exposed to high relative humidity.
(c) Biological cross-contamination: Example: Improper cleaning of equipment, unclean equipments used for the manufacturing process.
Types of Food Contaminants:
(a) Biological contaminants: Microbial contaminants are responsible for foodborne diseases that are caused by bacteria, viruses, fungi, parasites, biological toxins, etc.
e.g.: Seafood toxins, Mushroom toxins.
(b) Chemical contaminants: Chemical substances that are responsible for food-borne illness.
e.g.: Toxic metals, Pesticides, etc.
(c) Physical contaminants: Any foreign substances that are accidentally observed in the food.
e.g.: Hair, wire, dust, sand particles, etc.
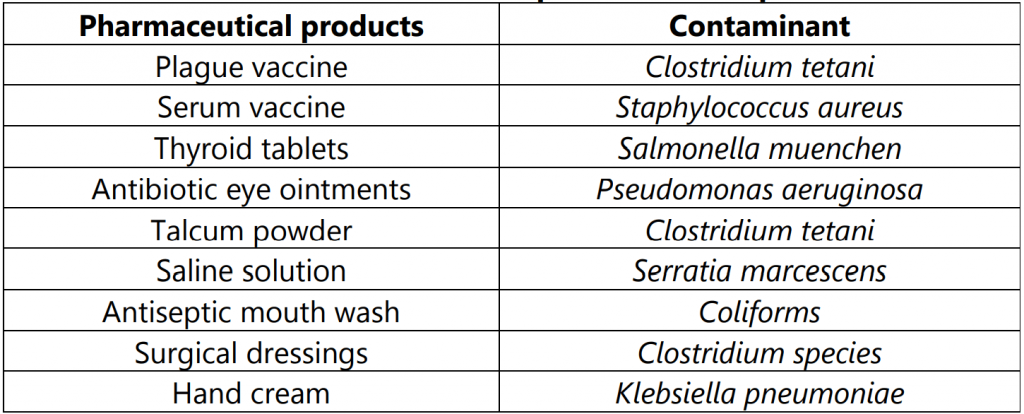
Some of the examples of contaminants for pharmaceutical products are given in Table.1.
Make sure you also check our other amazing Article on : Spoilage