Preparation and Standardization of Sulfuric Acid
Aim: To perform the standardization of sulfuric acid.
Requirements:
- Apparatus: Beaker, Funnel, Pipette, Burnette.
- Chemicals: Sulfuric acid, Anhydrous sodium carbonate, Phenolphthalein solution.
Theory:
Methyl orange is one of the indicators commonly used in titrations. In an alkaline solution, methyl orange is yellow and the structure is:
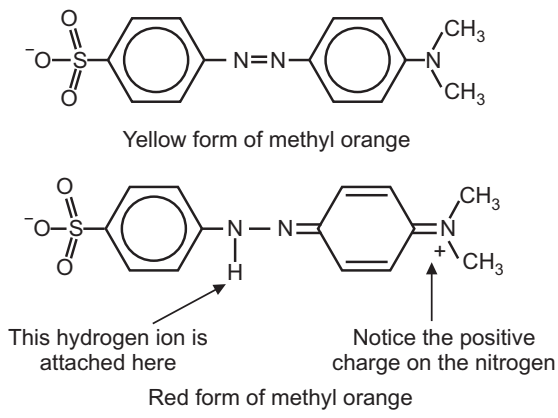
Procedure:
(A) Preparation of Sulfuric acid (1 M): Add slowly, with stirring, 30 ml of sulphuric acid to about 1000 ml of water, allow to cool at 25°C.
(B) Standardisation: Weigh about 1.5 g of anhydrous sodium carbonate powder accurately in a watch glass. Transfer the solid totally into a 250 ml beaker where about 50 cm3 distilled water is already filled. Wash the watch glass thoroughly using a washing bottle and transfer all the washings into the 250 ml beaker. Add more water to dissolve the remaining solid. Use a glass rod to stir the solution to facilitate the dissolving process. Transfer the solution carefully to a 250 ml volumetric flask using a filter funnel and a glass rod. Rinse the beaker, glass rod, and inner surface of the funnel with water and transfer all the washings to the volumetric flask. Repeat this process two or three times. Make up the solution to 250 ml in the volumetric flask by adding water. Pipette 25 ml of the sodium carbonate solution into a conical flask, add a few drops of methyl orange indicator, and titrate against the sulfuric acid solution. Determine the molarity of a sulfuric acid solution. Color change : yellow to reddish-orange.
Observation Table of Preparation and Standardization of Sulfuric Acid
| Sr. No. | Parameters | Reading |
| 1. | Burette solution | Sulfuric acid solution |
| 2. | Conical flask solution | Anhydrous sodium carbonate, water |
| 3. | Indicator | Methyl orange |
| 4. | Endpoint | Yellow to orange |
| Burette reading B.R. (ml) | Trial 1 | Trial 2 | Trial 3 | Average (BR) |
Reaction:
Na2CO3(aq) + H2SO4(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
Calculations:
(A) Factor Calculations:
- Molecular weight of Na2CO3 = 105.98 g.
From the reaction:
- 1 mol of H2SO4 is equivalent to 1 mol of Na2CO.
- Hence, 1000 ml of 1 M H2SO4 is equivalent to 105.98 g of Na2CO3.
- So, 1 ml of 0.5 M H2SO4 is equivalent to 0.05299 of Na2CO3.
(B) Determination of Normality:
- 1 ml of 0.5 M H2SO4 is equivalent to 0.05299 of Na2CO3.
- So, (B.R) ml of A M H2SO4 is equivalent to 1.5 g of Na2CO3.
- Hence, A = 1 × 1.5/BR × 0.05299.
Result:
The normality of given H2SO4 is found to be “A” N.
Make sure you also check our other amazing Article on : Standardization of Sodium Hydroxide
