Biphasic liquids such as suspensions and emulsions are unique dosage forms because many of their properties are due to the presence of a boundary region between two phases. In suspensions, a liquid and an insoluble solid meet to form an interface. In the case of emulsions, two immiscible liquids, usually oil and water, form an interface.
Formulation of Suspensions
Table of Contents
1. Wetting agents: They are added to disperse solids in a continuous liquid phase. Example: Polysorbate 80, 20, span, etc.
2. Suspending agents: They are added to flocs the drug particles.
3. Thickeners: They are added to increase the viscosity of the suspension. Example: gaur gum, xanthan gum.
4. Buffers and pH adjusting agents: They are added to stabilize the suspension to the desired pH range.
5. Colouring agents: They are added to impart the desired color to suspension and improve elegance.
6. Preservatives: They are added to prevent microbial growth.
Preparation of Suspensions
Step 1: Suspensions are prepared by grinding the insoluble materials in the mortar to a smooth paste with a vehicle containing the wetting agent.
Step 2: All soluble ingredients are dissolved in the same portion of the vehicle and added to the smooth paste to step 1 to get slurry.
Step 3: The slurry is transformed into a graduated cylinder, the mortar is rinsed with a successive portion of the vehicle.
Step 4: Decide whether the solids are
- Suspended in a structured vehicle
- Flocculated
- Flocculated and then suspended
Add the vehicle containing the suspending agent (or) flocculating agent.
Step 5: Make up the dispersion to the final volume. Thus suspension is prepared.
Stability of Suspension
Factors that contribute to the appreciable stability of a suspension include:
1. Small Particle Size:
- The reduced size of the dispersed particle increases the total surface area of the solid.
- The greater the degree of subdivision of a given solid the larger the surface area.
- The increase in surface area means also an increase in the interface between the solids and liquids leading to an increase in viscosity of a system.
2. Increasing the Viscosity:
- Increased viscosity of the continuous phase can lead to the stability of suspensions.
- This is so because the rate of sedimentation can be reduced by the increase in viscosity. Viscosity increase is brought about by the addition of thickening agents to the external phase.
- It is important to note that the rate of release of a drug from a suspension is also dependent on viscosity.
3. Temperature:
- Another factor that negatively affects the stability and usefulness of pharmaceutical suspensions is the fluctuation of temperature. Temperature fluctuations can lead to caking and claying.
Formulation of Emulsions
Emulsifying Agents (Emulsifiers): An emulsifying agent is a material that enhances the stability of an emulsion (i.e. Prevention of coalescence and reducing creaming).
The ideal emulsifying agent is colorless, odorless, tasteless, non-toxic, non-irritant, and able to produce stable emulsions at low concentrations.
Examples of Emulsifying Agents:
1. Carbohydrate Materials: Acacia, Tragacanth, Agar, Pectin. o/w emulsion.
2. Protein Substances: Gelatin, Egg yolk, Caesin o/w emulsion.
3. High Molecular Weight Alcohols: Stearyl Alcohol, Cetyl Alcohol, Glyceryl Monostearate o/w emulsion, cholesterol w/o emulsion.
4. Wetting Agents:
- Anionic, Cationic, Nonionic
- o/w emulsion -w/o emulsion
5. Finely divided solids: Bentonite, Magnesium Hydroxide, Aluminum Hydroxide o/w emulsion.
Tests for Identification of Emulsion Type:
- Dilution test (Miscibility test)
- Staining test (Dye solubility test)
- Conductivity measurement
- Fluorescence test
Preparation of Emulsions
The methods commonly used to prepare emulsions can be divided into two categories:
1. Trituration Method:
This method consists of the dry gum method and the wet gum method.
(i) Dry Gum Method: In this method, the oil is first triturated with gum with a little amount of water to form the primary emulsion. The trituration is continued till a characteristic ‘clicking’ sound is heard and thick white cream is formed. Once the primary emulsion is formed, the remaining quantity of water is slowly added to form the final emulsion.
4:2:1 formula 4 parts (volumes) of oil 2 parts of water 1 part of the gum
(ii) Wet Gum Method: As the name implies, in this method first gum and water are triturated together to form a mucilage. The required quantity of oil is then added gradually in small proportions with thorough trituration to form the primary emulsion. Once the primary emulsion has been formed remaining quantity of water is added to make the final emulsion.
4:2:1 formula 4 parts (volumes) of oil 2 parts of water 1 part of gum Homogenizer /Mortar and Pestle.
2. Bottle Method:
- This method is employed for preparing emulsions containing volatile and other non-viscous oils. Both dry gum and wet gum methods can be employed for the preparation.
- As volatile oils have a low viscosity as compared to fixed oils, they require a comparatively large quantity of gum for emulsification.
- In this method, oil or water is first shaken thoroughly and vigorously with the calculated amount of gum. Once this has been emulsified completely, the second liquid (either oil or water) is then added all at once and the bottle is again shaken vigorously to form the primary emulsion. More water is added in small portions with constant agitation after each addition to produce the final volume.
Stability of Emulsion
- An emulsion is said to be stable if it remains as such after its preparation, i.e. the dispersed globules are uniformly distributed throughout the dispersion medium during its storage. The emulsion should be chemically stable and there should not be any bacterial growth during its shelf life.
- Emulsion instability may either be reversible or irreversible and manifest in the following ways:
- Cracking (irreversible instability)
- Flocculation
- Creaming
- Phase inversion
1. Cracking: Cracking means the separation of two layers of dispersing and continuous phase, due to the coalescence of disperse phase globules which are difficult to redisperse by shaking.
Cracking may occur due to the following reasons:
- By the addition of emulsifying agent of the opposite type
- By decomposition or precipitation of emulsifying agent
- By the addition of common solvent
- By microorganisms
- Change in temperature
- By creaming
2. Flocculation: In the flocculated state the secondary interaction (Van der Waals’ forces) maintains the droplets at a defined distance of separation. Application of shearing stress to the formulation (shaking) will redisperse these droplets to form a homogeneous formulation. Although flocculation may stabilize the formulation, there is also the possibility that the close location of droplets would enable droplet coalescence to occur if the mechanical properties of the interfacial film are compromised.
3. Creaming: Creaming may be defined as the upward movement of dispersed globules to form a thick layer at the surface of the emulsion. Creaming is a temporary phase because it can be re-distributed by mild shaking or stirring to get again a homogenous emulsion.
The factors affecting creaming are described by Stoke’s law:
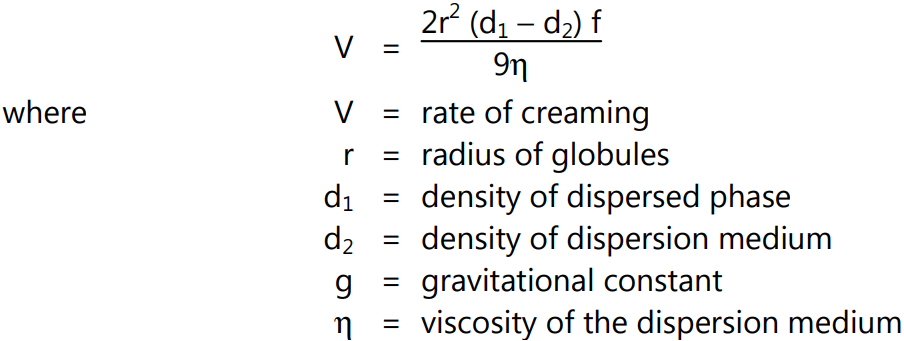
Following approaches can be used for decreasing creaming:
- Radius of globules
- The difference in density of dispersed phase and continuous phase
- The viscosity of the dispersion medium
- Storage condition
4. Phase inversion:
Phase inversion means the change of one type of emulsion into other types, i.e. oil in water emulsion changes into the water in oil type and vice-versa.
Due to the following reasons, the phase inversion takes place:
- By the addition of an electrolyte
- By changing the phase-volume ratio
- By temperature change
- By changing the emulsifying agent
The phase inversion can be minimized by keeping the concentration of disperse phase between 30 to 60 %, storing the emulsion in a cool place, and using a proper emulsifying agent’s inadequate concentration.
Packaging, Labeling, and Storage of Emulsions
- Depending on the use, emulsions should be packed in suitable containers. Emulsions meant for oral use are usually packed in well-filled bottles having an air tight closure
- Light-sensitive products are packed in amber-colored bottles.
- For viscous emulsions, wide-mouth bottles should be used. The label on the emulsion should mention that these products have to be shaken thoroughly before use.
- External use products should mention on their label that they are meant for external use only.
- Emulsions should be stored in a cool place but refrigeration should be avoided as this low temperature can adversely affect the stability of preparation.
Selection of Method: Liquid orals are filled into containers by different methods. The method selected for filling depends on the following;
- Characteristics of liquid
- Viscosity
- Surface tension
- Foam producing qualities
- Compatibility with the materials of construction of the filling machine
- The type of package
- The required production output
Methods of Filling
1. Gravimetric filling method
2. Volumetric filling method
3. Constant level filling method
(a) Vacuum filling
(b) Gravity-vacuum filling
(c) Pressure-vacuum filling
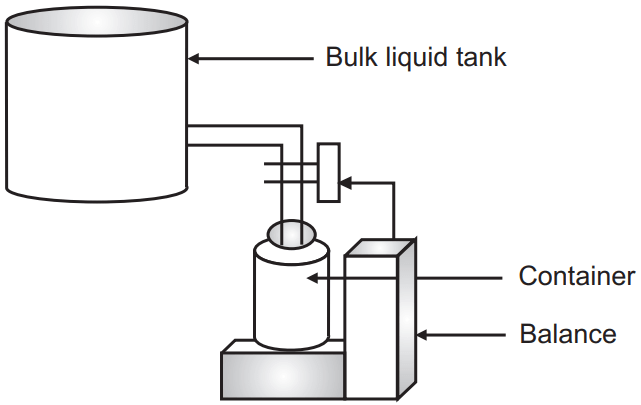
1. Gravimetric Filling Method: In this method liquid from the bulk liquid tank is allowed to flow into the containers up to a given weight.
Advantage: This is a useful technique for filling large quantities of the preparation and for highly viscous products.
Disadvantage: This method is not suitable for high-speed production rates and automatic equipment.
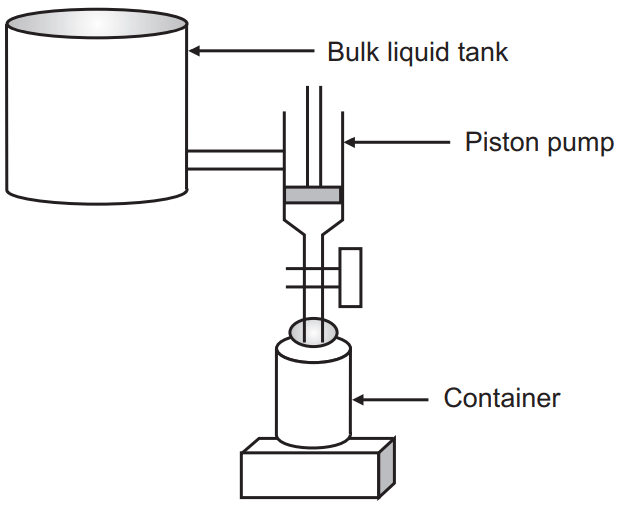
2. Volumetric Filing Method: In this method, the liquid is filled by positive displacement piston action. The filling station is equipped with a measuring piston and cylinder. The quality of the liquid to be filled is measured by the stroke of the piston.
Advantage: This type of arrangement can fill accurately to within a fraction of a milliliter.
Disadvantages:
- Highly viscous liquids may seize the piston resulting in either loss of fill accuracy or line breakdown.
- Very low viscous liquids flow very fast. This leads to dripping from the filling piston resulting in fill inaccuracies. Solution: The problems can be controlled by proper engineering of the filling equipment.
Containers of uniform dimensions must be used for filing to have a uniform appearance. Otherwise, even though the fill amount is accurate, the fill height varies inversely with the container capacity. An oversized package appears to have an insufficient fill, whereas an undersized package appears to have an excessive fill.
3. Constant Level Filling Method: In this method, the container is used as the means for controlling the fill of each unit. The full amount is varied by adjusting the height to which the container is filled.
Limitation: Any dimensional variations in the containers result in comparable variations in the net fill per unit.
The filling machines make use of a siphon principle. In addition, a high-pressure difference is created between the liquid discharge nozzle and the constant level overflow system.
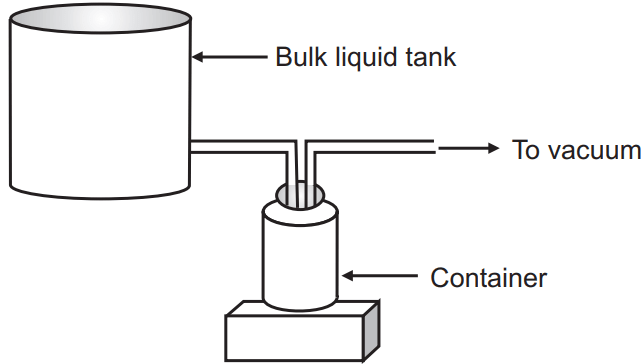
(a) Vacuum filling: A container is connected to the bulk liquid tank through the vacuum head. The connections must be air-tight. The container, on the other hand, is connected to a vacuum pump. A vacuum is then developed within the container. The developed vacuum causes the liquid to flow from the bulk liquid tank to the container. The liquid level rises until it reaches the vacuum tube, which is positioned at the desired constant level. If the excess liquid is drawn through the vacuum tube, from there the liquid is recycled to the bulk liquid tank.
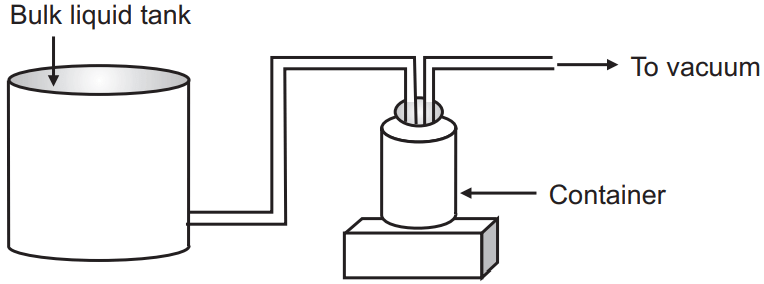
(b) Gravity-vacuum filling: In this method, the bulk liquid tank has placed a level above the container. Connections are made air-tight. Vacuum is applied for filling. In addition, the gravitational force also acts to aid filling.
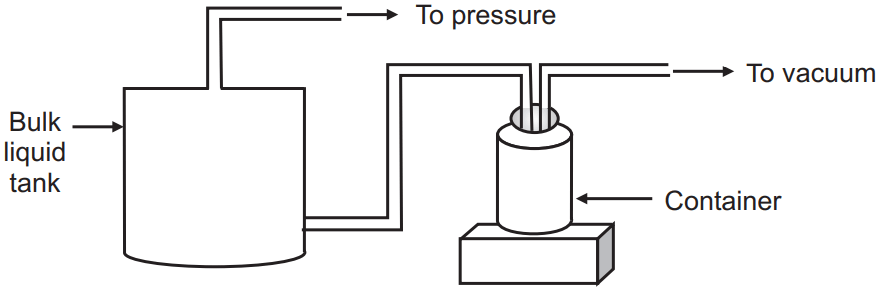
(c) Pressure-vacuum filling: Here also connections are made air-tight. The container is connected to a vacuum pump and the bulk liquid tank is connected to the positive pressure-inducing pump. Vacuum in the container and positive pressure above the bulk liquid synergistically act to fill the liquid.
Advantage: Suitable for high viscous liquids.
Foam production – Problem: Foam is produced during the filling with all these methods. This is a severe problem when high production rates are required.
Solution: The problem of foam formation cannot be solved. However, the intensity of the problem can be decreased by any of the following techniques;
(a) Using equipment that minimizes product turbulence.
(b) Using closed system filling equipment that prevents the entry of air or other gases which participate in the formation of foam.
(c) Using mechanical defoaming devices.
(d) Using low filling rates.
Package
Most liquid orals are packaged in either amber or flint glass containers with plastic or metal caps. Glass is generally inert to aqueous solutions in the pH range appropriate for oral liquids. But cap and liner may react. Plastic caps may undergo stress cracking in contact with some liquids. Metal caps may undergo corrosion. Therefore compatible closures must be selected on an individual basis.
Make sure you also check our other amazing Article on : Considerations of Syrups