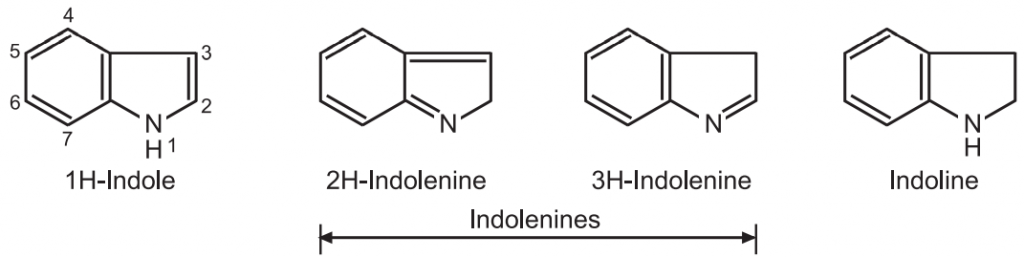
Synthesis and Reactions of Indole: Indole is an aromatic heterocyclic compound consisting of a benzene ring fused to a pyrrole ring. it is a white solid having a melting point of 52-54°C. Indole was first obtained in 1866 by zinc dust distillation of oxindole. The tautomeric forms of indole are known as indolenines.
Chemical Synthesis of Indole
(1) Aniline via vapor-phase reaction with ethylene glycol in the presence of catalyst gives indoles.
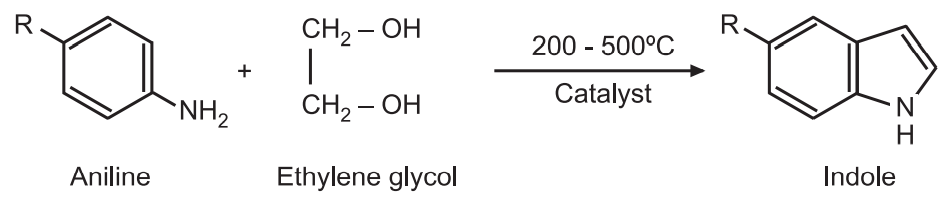
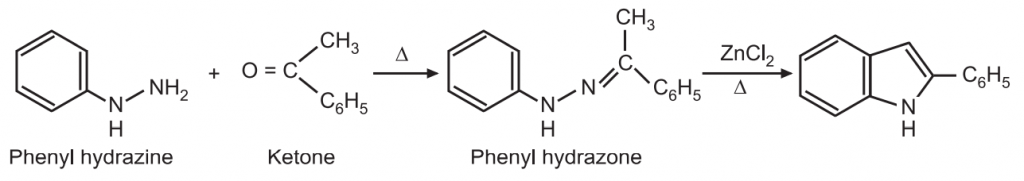
(2) Fischer-Indole synthesis: This method was developed in 1883 by Emil Fischer. It is used to synthesize 2- and/or 3-substituted indoles. It consists of heating an arylhydrazine with an aldehyde or ketone, followed by acid-catalyzed rearrangement of resulting arylhydrazone with a loss of ammonia give an indole.
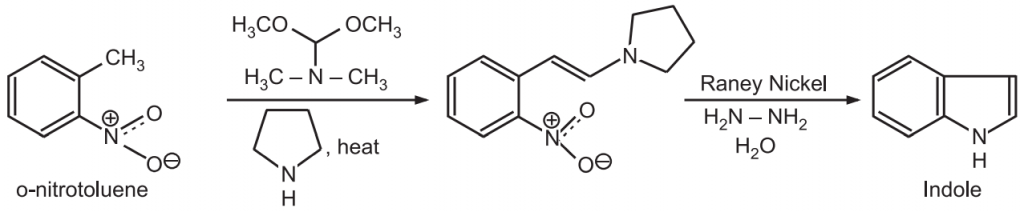
(3) Leimgruber-Batcho indole synthesis: In this reaction, o-nitrotoluence reacts with pyrrolidine in the presence of N, N-dimethyl formamide dimethyl acetal (DMFDMA) to give an enamine. This enamine undergoes reductive cyclization to give an indole.
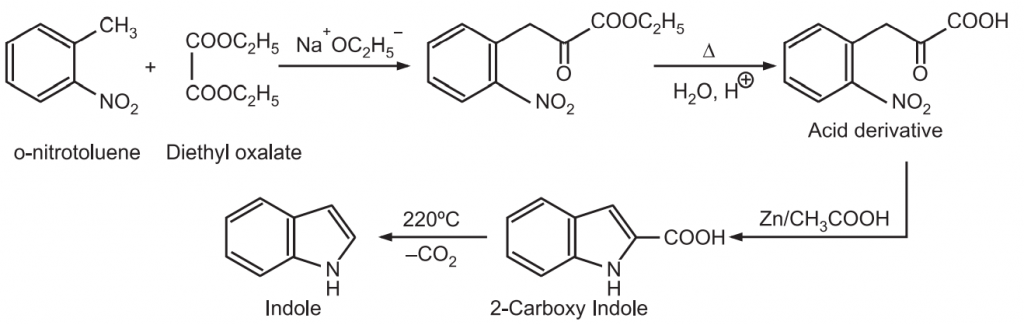
(4) Reissert synthesis: The methyl group ortho to nitro on a benzene ring is acidic enough to get condensed with diethyl oxalate in the presence of sodium ethoxide. After hydrolysis, the resulting acid undergoes reductive cyclization to give an indole.
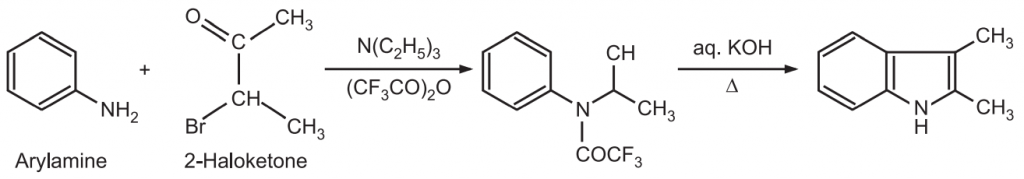
(5) Bichler synthesis: An arylamine is treated with a 2-halo ketone to give α-arylaminoketone which on heating with a strong acid or zinc chloride undergoes cyclization to give an indole.
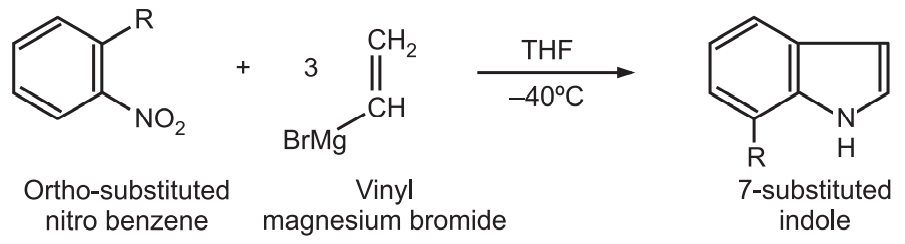
(6) Bartoli synthesis: In this method, o-substituted nitrobenzene is treated with three moles equivalents of vinyl magnesium bromide to give 7-substituted indoles.
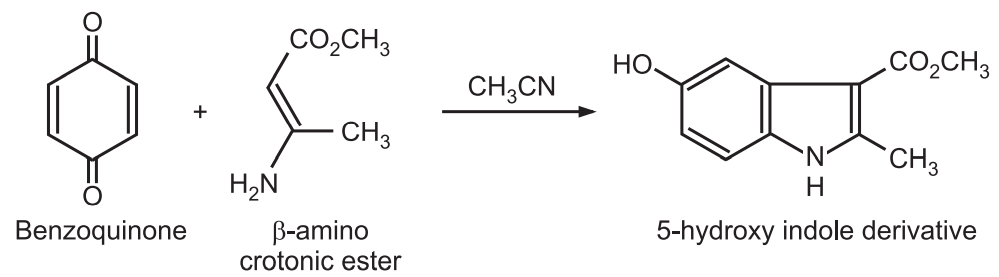
(7) Nenitzescu synthesis: Costin Nenitzescu reported this method in 1929. In this method, benzoquinone reacts with the β-aminocrotonic ester to give 5-hydroxy indole derivatives.
(8) Sugasawa synthesis: Arylamine undergoes Friedel-Crafts acylation using nitrile and boron trifluoride to give 2-amino chloroacetophenone.
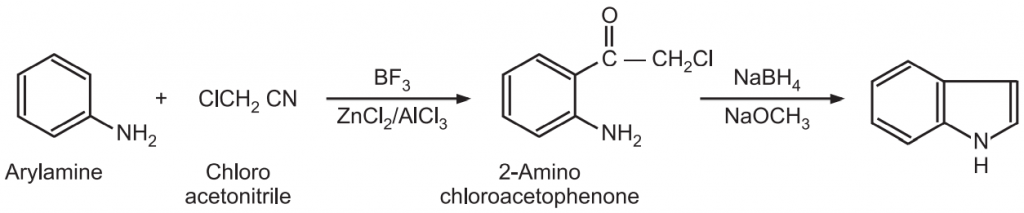
It is followed by reductive cyclization of the intermediate using NaBH4 to give 2, 3-unsubstituted indole derivatives. This reaction is not suitable for anilines with strong electron-withdrawing substituents.
Chemical Reactions of Indoles
Indoles are aromatic heterocyclic compounds where the ring nitrogen atom is not basic. Indoles are very weak bases.
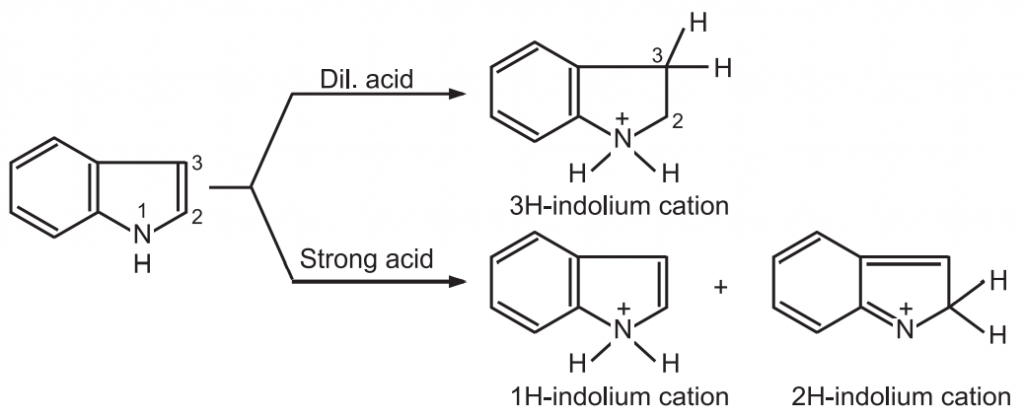
(1) Protonation:
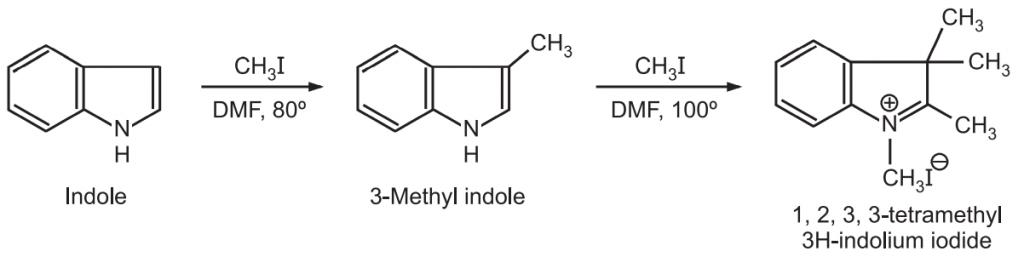
(2) Alkylation: The lone pair of electrons on ring N-atom is a part of the aromatic sextet and not available free and exclusively at the nitrogen atom. Hence, indoles do not react with alkyl halides at room temperature. However, indole reacts with methyl iodide in DMF at about 80°C to give 3-methyl indole. As the temperature is gradually raised above 100°C, 1, 2, 3, 3-tetramethyl-3H-in dolium iodide is formed.
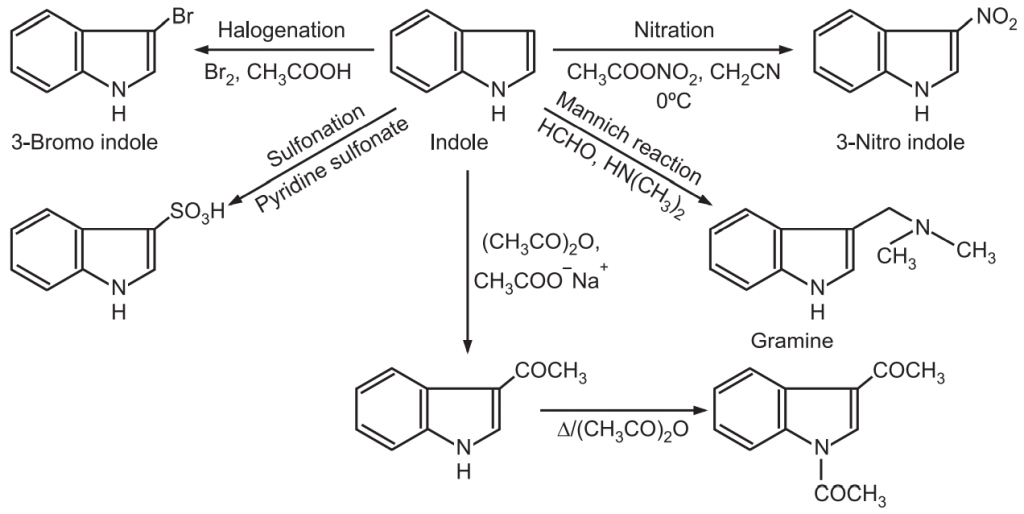
(3) Electrophilic substitution: Indole itself is an π-electron excessive system. Since electron density is higher on carbon atoms in the pyrrole part of indole, electrophilic substitution readily occurs in the heterocyclic ring in the following preference order.
C3 – atom > N-atom > C2 – atom
When positions 3- and 2- are occupied, the electrophile attacks in the benzene ring. The attack of electrophile at position-3 generates carbocation which efficiently uses electron lone pair on N-atom and does not disturb the aromaticity of benzene ring. While electrophile attack of position-2 generates carbocation, which disrupts the aromatic character by delocalizing the positive charge over the benzene ring.
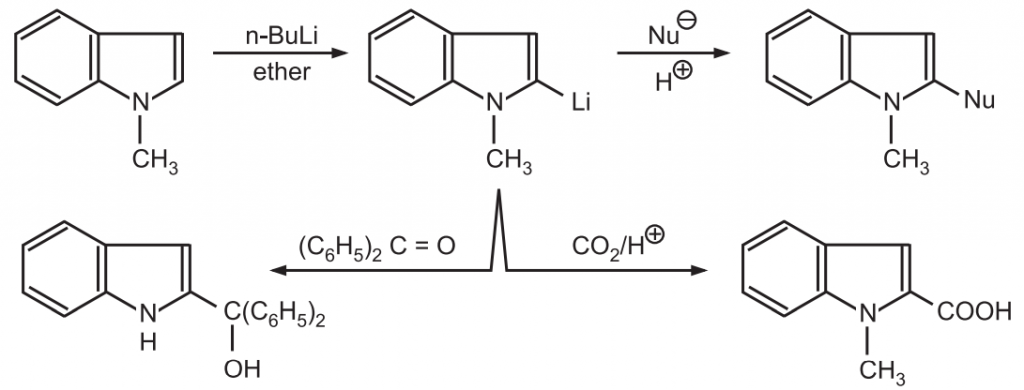
(4) Nucleophilic substitution: Indoles undergo very few nucleophilic substitution reactions. These reactions do not occur in indoles by direct displacement of the ring-bound substituent. Initially, the indole is deprotonated by readily displaceable nucleophilic reagents such as n-BuLi. The nucleophile then attacks at the C-2 position by displacing the Li-atom.
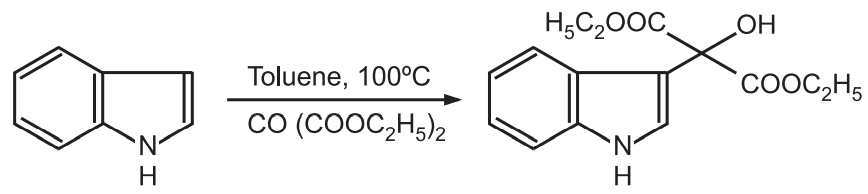
(5) Reaction with aldehydes and ketones: Under acid-catalyzed conditions, indoles react with aldehydes and ketones to give indol-3-ylcarbinols.
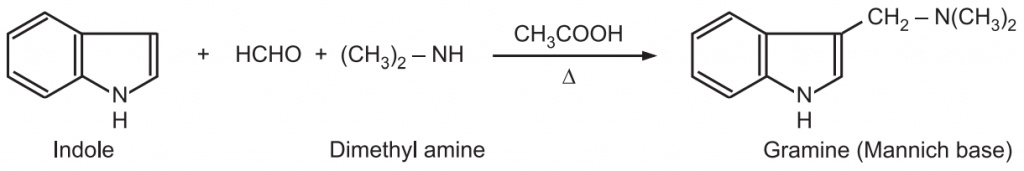
(6) Mannich reaction: Indole reacts with a mixture of formaldehyde and dimethylamine in acetic acid to give Mannich base at C3-position.
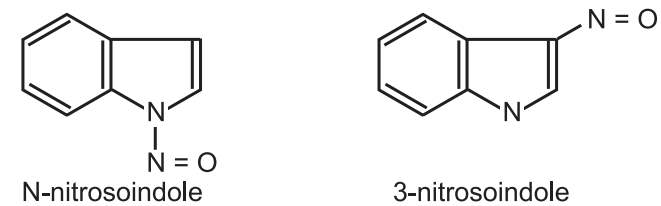
(7) Diazo-coupling and nitrosation: In a base-catalyzed process, indoles react with nitrosating agents to give N-nitroso- and 3-nitroso indoles.
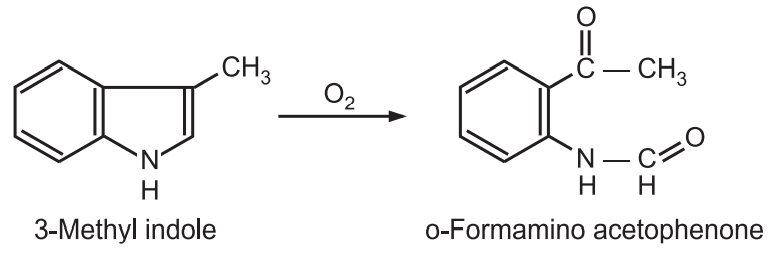
(8) Oxidation: In the presence of air and light, indole is auto-oxidized to a resinous material. A methyl substituent at C3-position in indole stabilizes the ring and gives o-form amino acetophenone through cleavage of indole 2,3-double bond. When 3-methyl indole is oxidized using oxygen, ozone, sodium hypoiodate, or perbenzoic acid.
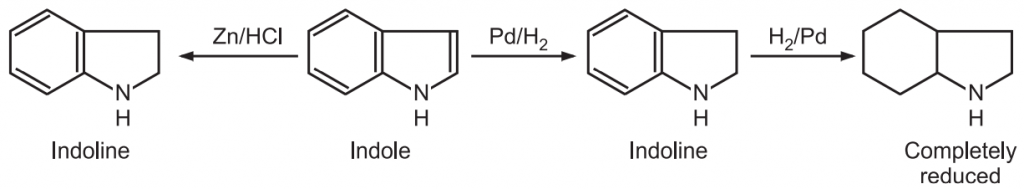
(9) Reduction: The indole ring can be reduced selectively in the benzenoid or heterocyclic ring. For example, zinc/HCl converts indole into indoline. Such conversion also occurs under catalytic reduction of indole in an acidic medium.
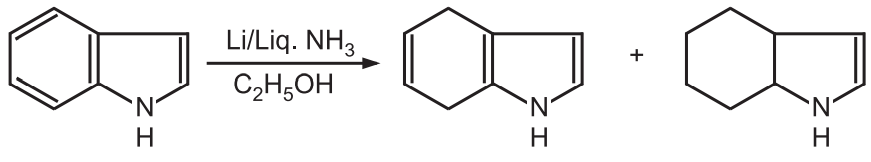
While lithium/liquid ammonia reduces the benzene ring only.
Application in Drug Synthesis
Indole is a core part of the structure of serotonin (neurotransmitter, autacoid), vinblastine (anti-cancer), indomethacin (NSAIDs), besipirdine (effective in the treatment of Alzheimer’s disease), fendosal (NSAIDs), Ondansetron (antiemetic in cancer chemotherapy) brasserie (anticancer), melatonin (hormone), etc.
Make sure you also check our other amazing Article on : Synthesis and Reactions of Isoquinoline