Tetrabutyl Ammonium Hydroxide (0.1M)
Molecular formula: (C4H9)4 N+OH−
Molecular weight: 259.48
Preparation: Dissolve 40 g of tetra butyl ammonium iodide in 90 mL of dehydrated methanol in a glass stoppered flask. Place the flask in an ice bath. Add 2 g of powdered silver oxide, insert the stopper, and agitate vigorously for 1 hour. Centrifuge a few mL, and test the supernatant liquid for iodides [acidify with dil. HNO3 and add AgNO3, a yellow precipitate of AgI precipitates]. If the test is positive, add 2g of silver oxide additionally and continue to stand for 30 minutes with intermittent agitation. When the iodide completely reacted, filter the solution through a sintered glass filter. Rinse the flask and filter with three quantities, each of 50 mL of anhydrous toluene. Add the washing to the filtrate and dilute to 1000 mL with anhydrous toluene. Flush the solution for 10 minutes with dry carbon dioxide-free nitrogen. Store the solution in a container protected from CO2 and moisture.
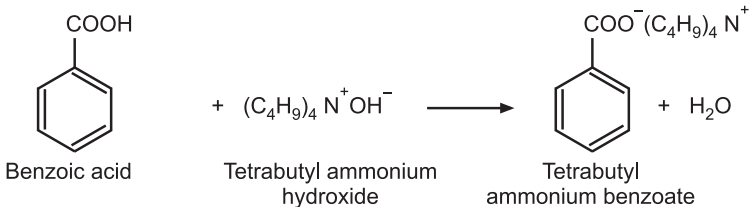
Standardization: Weigh accurately about 0.4 g of benzoic acid, dissolve in 80 mL of dimethyl formamide, add a few drops of 1% solution of thymol blue in dimethyl formamide as an indicator and titrate with tetra butyl ammonium hydroxide solution to give blue color as the endpoint. Protect the solution from atmospheric CO2 throughout the titration. Perform a blank determination and make any necessary corrections.
Reaction:
Factor Calculation:
1 mole of (C4H9)4 N+OH− ≅ 1 mole of benzoic acid
1000 mL of 1M (C4H9)4 N+OH− ≅ 122.12 g of C6H6COOH
1 mL of 0.1M (C4H9)4 N+OH− ≅ 0.01221 g of C6H6COOH
Make sure you also check our other amazing Article on: How do you make 1M sodium methoxide?
