The transdermal drug delivery system (TDDS) is one of the systems lying under the category of controlled drug delivery, in which the aim is to deliver the drug through the skin at a predetermined and controlled rate. TDDS are adhesive drug-containing devices of defined surface area that deliver a predetermined amount of drug to the surface of intact skin at a programmed rate to reach the systemic circulation.
Transdermal delivery provides a leading-edge over injectables and oral routes by increasing patient compliance and avoiding first-pass metabolism, respectively. The transdermal route has vied with oral treatment as the most successful innovative research area in drug delivery, as oral treatment involves attainment and maintenance of drug concentration in the body within a therapeutically effective range by the introduction of a fixed-dose at regular intervals, due to which the drug concentration in the body follows a peak and trough profile, leading to a greater chance of adverse effects or therapeutic failure; a large amount of drug is lost in the vicinity of the target organ and close attention is required to monitor therapy to avoid overdosing. The limitations of the oral route can be overcome and benefits of intravenous drug infusion such as bypassing hepatic “first pass” hepatic elimination (HEPE) to maintain constant prolonged and therapeutic effective drug levels in the body can be closely duplicated, without its potential hazards, by transdermal drug administration through intact skin.
Permeation Through Skin
Table of Contents
Skin: The Largest Organ
The skin is the largest organ of the human body which covers a surface area of approximately 2 sq. m. and receives about one-third of the blood circulation through the body. It serves as a permeability barrier against the transdermal absorption of various chemical and biological agents. It is one of the most readily available organs of the body with a thickness of a few millimeters (2.97 – 0.28 mm) which,
- Separates the underlying blood circulation network from the outside environment.
- Serves as a barrier against physical, chemical, and microbiological attacks.
- Acts as a thermostat in maintaining body temperature.
- Plays role in the regulation of blood pressure.
- Protects against the penetration of UV rays.
- Skin is a major factor in determining the various drug delivery aspects like permeation and absorption of drugs across the dermis. The diffusional resistance of the skin is greatly dependent on its anatomy and ultrastructure.
Anatomy of Skin
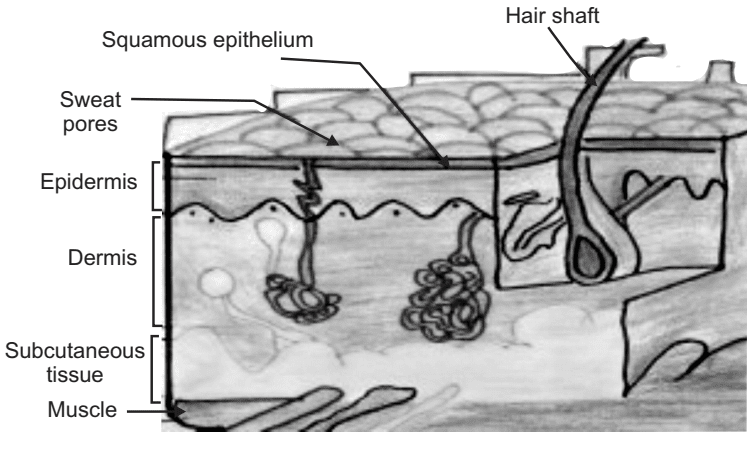
The structure of human skin (Fig.1) can be categorized into four main layers:
- The epidermis.
- The viable epidermis.
- A non-viable epidermis (Stratum corneum).
- The overlying dermis.
The innermost subcutaneous fat layer (Hypodermis).
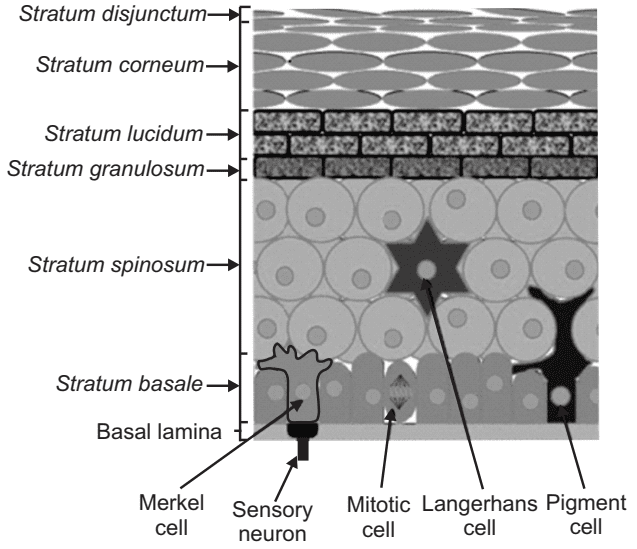
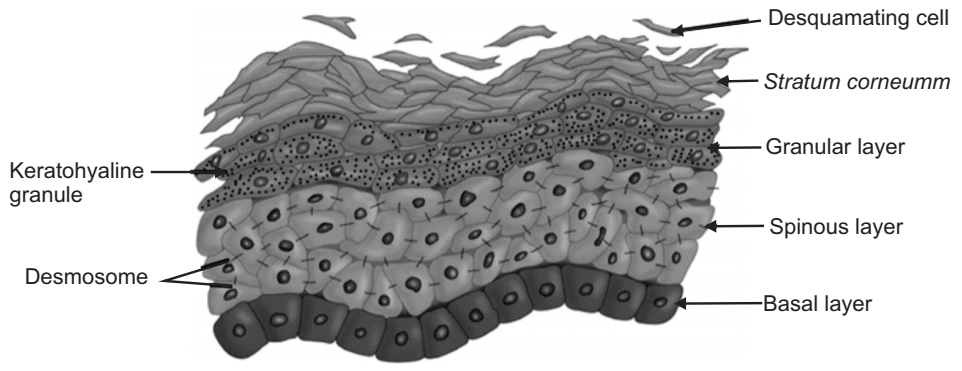
The Epidermis The epidermis is a continually self-renewing, stratified squamous epithelium covering the entire outer surface of the body and primarily composed of two parts: the living or viable cells of the malpighian layer (viable epidermis) and the dead cells of the stratum corneum commonly referred to as the horny layer. The viable epidermis is further classified into four distinct layers as shown in Fig.2.
- Stratum lucidum
- Stratum granulosum
- Stratum spinosum
- Stratum basale
Stratum Corneum
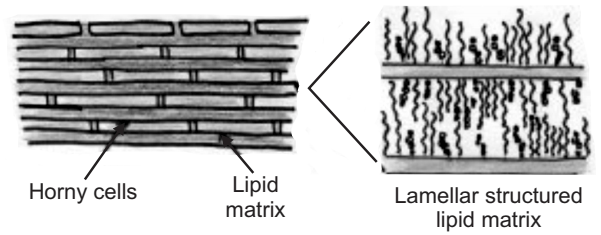
This is the outermost layer of skin also called a horny layer. It is the rate-limiting barrier that restricts the inward and outward movement of chemical substances. The barrier nature of the horny layer depends critically on its constituents: 75-80% proteins, 5-15% lipids, and 5-10% ondansetron material on a dry weight basis.
Stratum corneum is approximately 10 mm thick when dry, but swells several times when fully hydrated. It is flexible but relatively impermeable. The architecture of the horny layer (Fig.3) may be modeled as a wall-like structure with protein bricks and lipid mortar. It consists of horny skin cells (corneocytes) which are connected via desmosomes (protein-rich appendages of the cell membrane). The corneocytes are embedded in a lipid matrix which plays a significant role in determining the permeability of substance across the skin.
Viable Epidermis
This is situated beneath the stratum corneum and varies in thickness from 0.06 mm on the eyelids to 0.8 mm on the palms. Going inwards, it consists of various layers as stratum lucidum, stratum granulosum, stratum spinosum, and the stratum basale. In the basal layer, mitosis of the cells constantly renews the epidermis and this proliferation compensates for the loss of dead horny cells from the skin surface. As the cells produced by the basale layer move outward, they alter morphologically and histo-chemically, undergoing keratinization to form the outermost layer of stratum corneum shown in Fig.4.
Dermis
The dermis is the layer of skin just beneath the epidermis which is a 3 to 5 mm thick layer and is composed of a matrix of connective tissues, which contains blood vessels, lymph vessels, and nerves. The cutaneous blood supply has an essential function in the regulation of body temperature. It also provides nutrients and oxygen to the skin, while removing toxins and waste products. Capillaries reach within 0.2 mm of the skin surface and provide sink conditions for most molecules penetrating the skin barrier. The blood supply thus keeps the dermal concentration of permeate very low, and the resulting concentration difference across the epidermis provides the essential driving force for transdermal permeation. In terms of transdermal drug delivery, this layer is often viewed as essentially gelled water, and thus provides a minimal barrier to the delivery of most polar drugs, although the dermal barrier may be significant when delivering highly lipophilic molecules.
Hypodermis
The hypodermis or subcutaneous fat tissue supports the dermis and epidermis. It serves as a fat storage area. This layer helps to regulate temperature, provides nutritional support and mechanical protection. It carries principal blood vessels and nerves to the skin and may contain sensory pressure organs. For transdermal drug delivery, the drug has to penetrate through all three layers and reach the systemic circulation.
Percutaneous Absorption
Before a topically applied drug can act either locally or systemically, it must penetrate through the stratum corneum. Percutaneous absorption is defined as, “penetration of substances into various layers of skin and permeation across the skin into the systemic circulation. Percutaneous absorption of drug molecules is of particular importance in the transdermal drug delivery system because the drug has to be absorbed to an adequate extent and rate to achieve and maintain uniform, systemic, therapeutic levels throughout use. In general, once drug molecule crosses the stratum corneal barrier, passage into deeper dermal layers and systemic uptake occurs relatively quickly and easily.
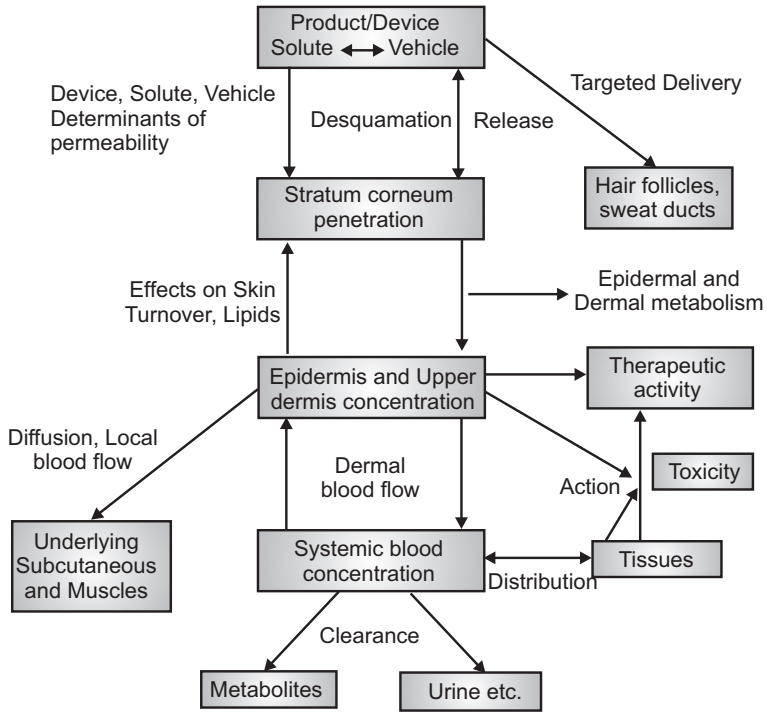
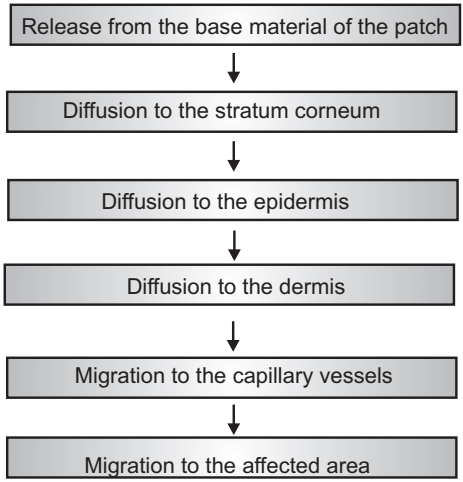
The release of a therapeutic agent from a formulation applied to the skin surface and its transport to the systemic circulation is a multistep process (Fig.5) that involves:
- Dissolution within and released from the formulation.
- Partitioning into the skin’s outermost layer, the stratum corneum (SC).
- Diffusion through the SC, principally via a lipidic intercellular pathway.
- Partitioning from the SC into the aqueous viable epidermis, diffusion through the viable epidermis and into the upper dermis, uptake into the papillary dermis (capillary system), and into the microcirculation.
Routes of Drug Penetration Through Skin
In the process of percutaneous permeation, a drug molecule may pass through the epidermis itself or may get diffuse through shunts, particularly those offered by the relatively widely distributed hair follicles and eccrine glands as shown in Fig.6. In the initial transient diffusion stage, drug molecules may penetrate the skin along with the hair follicles or sweat ducts and then be absorbed through the follicular epithelium and the sebaceous glands. When a steady-state has been reached, the diffusion through the intact Stratum corneum becomes the primary pathway for transdermal permeation.
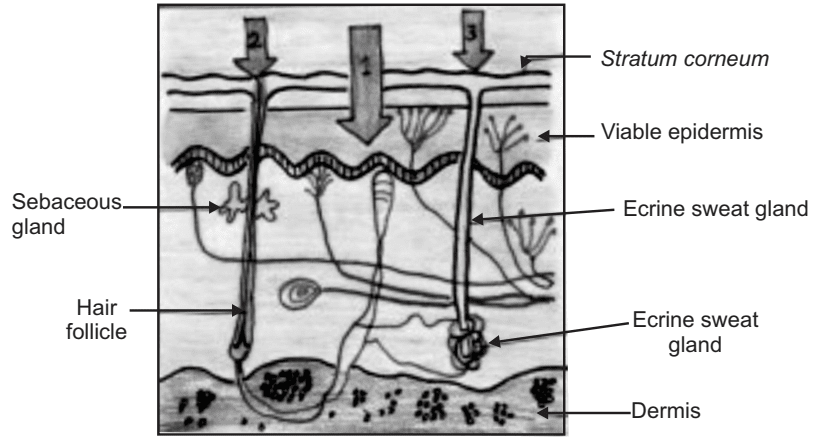
For any molecules applied to the skin, two main routes of skin permeation can be defined:
- Transepidermal route
- Transfollicular route
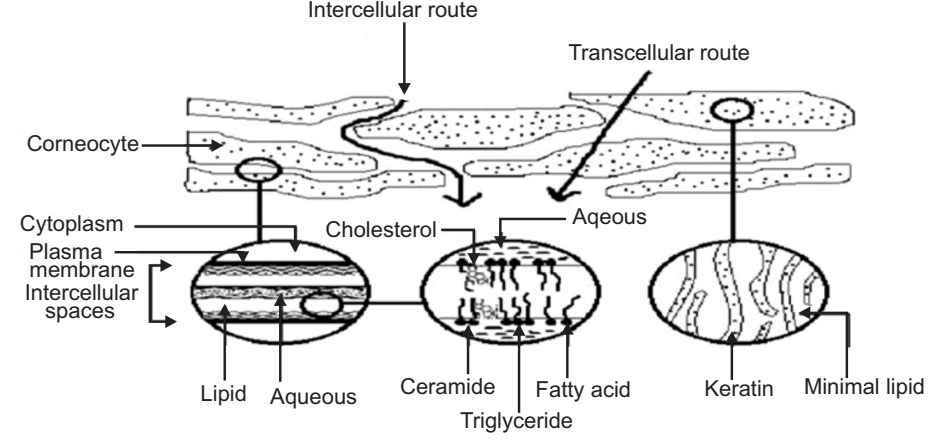
Transepidermal Route
In transepidermal transport, molecules cross the intact horny layer. Two potential micro routes of entry exist, the transcellular (or intracellular) and the intercellular pathway are shown in Fig.7.
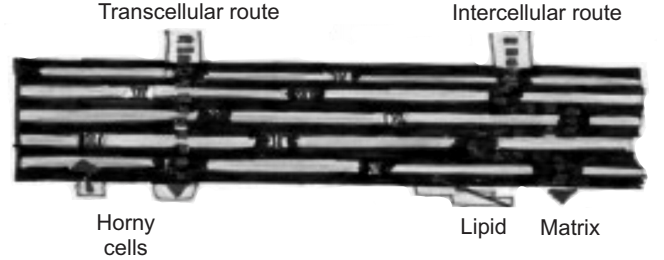
Both polar and non-polar substances diffuse via transcellular and intercellular routes by different mechanisms. The polar molecules mainly diffuse through the polar pathway consisting of “bound water” within the hydrated stratum corneum whereas, the non-polar molecules dissolve and diffuse through the non-aqueous lipid matrix of the stratum corneum. Thus, the principal pathway taken by a penetrant is decided mainly by the partition coefficient (log K). Hydrophilic drugs partition preferentially into the intracellular domains, whereas, lipophilic permeants (octanol/water log K > 2) traverse the stratum corneum via the intercellular route. Most molecules pass the stratum corneum by both routes.
Intercellular or Transcellular, Transfollicular Route (Shunt Pathway)
This route comprises transport via the sweat glands and the hair follicles with their associated sebaceous glands. Although these routes offer high permeability, they are considered to be of minor importance because of their relatively small area, approximately 0.1% area of the total skin. This route seems to be most important for ions and large polar molecules which hardly permeate through the stratum corneum.
Barrier Functions of the Skin
The top layer of skin is the most important function in maintaining the effectiveness of the barrier. Here the individual cells overlie each other and are tightly packed, preventing bacteria from entering and maintaining the water-holding properties of the skin. The stratum corneum mainly consists of the keratinized dead cell and water content is also less as compared to the other skin components. Lipids are secreted by the cells from the base layer of the skin to the top. These lipid molecules join up and form a tough connective network, in effect acting as the mortar between the bricks of a wall.
Kinetics of Transdermal Permeation
Transdermal permeation of a drug involves the following three steps:
- Sorption by stratum corneum.
- Penetration of drug through the viable epidermis.
- Uptake of the drug by the capillary network in the dermal papillary layer.
The rate of permeation across the skin is given by,
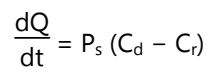
Where Cd and Cr are the concentrations of skin that penetrate in the donor compartment and the receptor compartment. Ps is the overall permeability coefficient of the skin tissues to penetrate.
This permeability coefficient is given by the relationship:
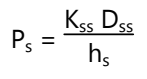
Where, Ks is the partition coefficient for the interfacial partitioning of the penetrate molecule from a solution medium or a transdermal therapeutic system onto the stratum corneum, Dss is the apparent diffusivity for the steady-state diffusion of the penetrate molecule through a thickness of skin tissues and hs is the overall thickness of skin tissues.
As Ks, Dss, and hs are constant under given conditions, the permeability coefficient (Ps) for a skin penetrate can be considered to be constant. From equation (1) it is clear that a constant rate of drug permeation can be obtained only when Cd >> Cr, i.e., the drug concentration at the surface of the stratum corneum (Cd) is consistently and substantially greater than the drug concentration in the body (Cr). Then the equation (1) becomes:
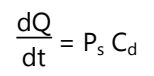
The rate of skin permeation (dQ/dt) is constantly provided the magnitude of Cd remains fairly constant throughout skin permeation. For keeping Cd constant, the drug should be released from the device at a rate (Rr) that is either constant or greater than the rate of skin uptake (Ra).
Since Rr is greater than Ra, the drug concentration on the skin surface (Cd) is maintained at a level equal to or greater than the equilibrium solubility of the drug in the stratum corneum (Cs). Therefore, a maximum rate of skin permeation is obtained and is given by the equation:
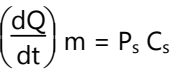
From the above equation, it can be seen that the maximum rate of skin permeation depends on the skin permeability coefficient (Ps) and its equilibrium solubility in the stratum corneum (Cs).
Stages in Drug Delivery in a Transdermal Patch
Following are the stages in drug delivery in a transdermal patch:
- Release of medicament from the vehicle.
- Penetration through the skin barriers.
- Activation of the pharmacological response.
The Transdermal patch effective therapy optimizes these steps as they are affected by three components, the drug, the vehicle, and the skin. Which represents the movement of drug molecules arising from, for example, a transdermal drug delivery system with a rate-controlling membrane illustrates the complexity of percutaneous absorption. Any drug particles must first dissolve so that molecules may diffuse towards the membrane within the patch. They penetrate partitions into the membrane, diffuse across the polymer, and partition into the skin adhesive. The molecules diffuse towards the vehicle/stratum corneum interface. They then partition into the stratum corneum and diffuse through it. Some drugs may bind at a depot site; the remainder permeates further, meets a second interface, and partitions into the viable epidermis. For a lipophilic species, this partition coefficient may be unfavorable, i.e., less than 1. Within the epidermis, enzymes may metabolize the drug or it may interact at a receptor site. After passing into the dermis, additional depot regions and metabolic sites may intervene as the drug moves to the capillary, partitions into its wall, and out into the blood for systemic removal. A fraction of the diffusing may partition into the subcutaneous fat to form a further depot. A portion of the drug can reach deep muscle layers, as illustrated by, for example, the efficacy of non-steroidal anti-inflammatory drugs. However, there are further complications. The following factors may be important: the nonhomogeneity of the tissues; the presence of lymphatics; interstitial fluid; hair follicles and sweat glands; cell division; cell transport to and through the stratum corneum; and cell surface loss. The disease, the healing process, the drug and vehicle components may progressively modify the skin barrier. As vehicle ingredients diffuse into the skin, cellular debris, sweat, sebum, and surface contaminants pass into the dermis, changing its physicochemical characteristics. Emulsions may invert or crack when rubbed in, and volatile solvents may evaporate.
Advantages of Transdermal Drug Delivery System
- Suitable for drug candidates with a short half-life and low therapeutic index.
- No first-pass effect.
- Reduction in dosing frequency.
- Minimization of daily intake of the drug.
- Reduction of fluctuations in plasma drug concentration.
- Improves patient compliance.
- Minimization of side effects.
- Simple and non-invasive.
- An alternate route for patients who are unable to take oral medications.
- Dose delivery unaffected by vomiting or diarrhea.
- Drug administration stops with patch removal.
Disadvantages of Transdermal Drug Delivery System
- The transdermal route of administration is unsuitable for drugs that irritate or sensitize the skin.
- Only relatively potent drugs are suitable for transdermal delivery due to the natural limits of drug entry by the skin’s impermeability.
- Technical difficulties with the adhesion of the systems to different skin types and under various environmental conditions.
- A constant concentration gradient is difficult to maintain.
Factors Affecting Transdermal Drug Delivery System
Physicochemical Properties of Drug
Following are the Physiochemical Properties of the Drug:
- Partition coefficient
- Molecular size
- Solubility/Melting point
- Ionization
- Diffusion Coefficient
Partition Coefficient
Drugs possess both water and lipid solubility. The ideal partition coefficient for intermediate transdermal delivery is log K1 − 3. For highly lipophilic drugs (log k < 3), the intracellular route is favorable, whereas, for hydrophilic drugs (log k ˂ 1), it is permeated via the transcellular route.
Molecular Size
The molecular size of the drug is inversely proportional to transdermal flux. The ideal molecular size of a drug molecule for transdermal delivery is ≤400.
Solubility/Melting Point
Most organic solutes have a high melting point and low solubility at normal temperature and pressure. Lipophilic drug permeates faster than hydrophilic substances, but they should also have aqueous solubility as needed in most topical formulations.
Ionization
Unionized drug permeates the skin according to the pH-Partition hypothesis.
Diffusion Coefficient
Penetration of drugs depends on the diffusion coefficient of the drug. At a constant temperature, the diffusion coefficient of the drug mainly depends on the properties of the drug, the diffusion medium, and their interaction.
Physicochemical Properties of Drug Delivery System
Following are the Physicochemical Properties of Drug Delivery System:
- Release characteristics.
- Composition of drug delivery system.
- Enhancement of Transdermal permeation.
Release Characteristics
The drug release mechanism mainly depends on drug molecules that are dissolved or suspended in the delivery system and on the interfacial partition coefficient or pH of the drug from the delivery system to the skin tissue. If the drug is easily released from the delivery system, the rate of transdermal permeation will be higher.
Composition of Drug Delivery System
The composition may not affect release properties but may affect its permeability functionality. For example, methyl salicylate is more lipophilic than parent acid, i.e. salicylic acid, and its percutaneous absorption is high when applied to the skin in a lipoidal vehicle.
Enhancement of Transdermal Permeation
The majority of drugs will not permeate into the skin for therapeutic use. Some enhancers are used for synergistic action without showing their properties (e.g. dimethyl sulphoxide, acetone, propylene glycol, and tetra-dihydro furyl alcohol).
Physiological Properties
Following are the Physiological and Pathological Conditions of Skin:
- Skin permeation barrier in neonate and infants
- Skin barrier properties in aged skin
- Race
- Skin temperature
Skin Barrier Properties in the Neonate and Young Infant
The skin surface of the newborn is slightly hydrophobic, relatively dry, and rough when compared to that of older infants. Stratum corneum hydration stabilizes by the age of 3 months.
Skin Barrier Properties in Aged Skin
There are some changes in the physiology of aged skin (465 years). The moisture content of human skin decreases with age. There is a destruction of the epidermal junction and consequently, the area available for transmission into the dermis is diminished.
Race
Racial differences between black and white skins have shown some anatomical and physiological functions of the skin. In black skin, there is increased intracellular permeation due to higher lipid content and higher electrical skin resistance levels when compared to whites, but this difference is not detected in stripped skin.
Skin Temperature
The human body maintains a temperature of 32°C–37°C across the skin. Hence, an increase in temperature leads to an increase in diffusion through the tissue.
Basic Components of TDDS
- Polymer matrix/drug reservoir
- Membrane
- Drug
- Permeation enhancers
- Pressure-sensitive adhesives (PSA)
- Backing laminates
- Release liner
- Other excipients like plasticizers and solvents
Polymer Matrix/Drug Reservoir
Polymers are the backbone of TDDS, which control the release of the drug from the device. A polymer matrix can be prepared by dispersion of drug in a liquid or solid-state synthetic polymer base. Polymers used in TDDS should have biocompatibility and chemical compatibility with the drug and other components of the system, such as penetration enhancers and PSAs. Additionally, they should provide consistent and effective delivery of a drug throughout the product’s intended shelf-life, and should be safe.
The following criteria should be preferred in selecting the polymer to be used in the transdermal system:
- Molecular weight, glass transition temperature, and chemical functionality of the polymer should be such that the specific drug diffuses properly and gets released through it.
- The polymer should be stable, non-reactive with the drug, easily manufactured and fabricated into the desired product, and should be inexpensive.
- The polymer and its degradation products must be non-toxic or non-antagonistic to the host.
- The mechanical properties of the polymer should not deteriorate excessively when large amounts of active ingredients are incorporated into it.
Membrane
A membrane may be sealed to the backing to form a pocket to enclose the drug-containing matrix or used as a single layer in the patch construction. The diffusion properties of the membrane are used to control the availability of the drug and/or excipients to the skin. For example, ethylene vinyl acetate, silicone rubber, polyurethane, etc. are used as a rate-controlling membrane.
Drug
For successfully developing a TDDS, the drug should be chosen with great care. Transdermal patches offer many advantages to drugs that undergo extensive first-pass metabolism, drugs with narrow therapeutic windows, or drugs with a short half-life, which cause non-compliance due to frequent dosing.
There are some examples of drugs that are suitable for the transdermal drug delivery system like; Nicardipine hydrochloride, Captopril, Atenolol, Metoprolol tartarate, Clonidine, Indapamide, Propranolol hydrochloride, Carvedilol, Verapamil hydrochloride, and Niterdipine, etc.
Permeation Enhancers
One long-standing approach for improving TDD uses penetration enhancers (also called sorption promoters or accelerants), which increase the permeability of the SC to attain higher therapeutic levels of the drug candidate.
Penetration enhancers interact with structural components of the SC thus, modifying the barrier functions, leading to increased permeability. Three pathways are suggested for drug penetration through the skin: polar, non-polar, and polar/non-polar. The enhancers act by altering one of these pathways. The key to altering the polar pathway is to cause protein conformational change of solvent swelling.
The key to altering the non-polar pathway is to alter the rigidity of the lipid structure and fluidize the crystalline pathway (this substantially increases diffusion). The fatty acid enhancers increase the fluidity of the lipid portion of the SC. Some enhancers (binary vehicles) act on both polar and non-polar pathways by altering the multi-laminate pathway for penetrants. The methods employed for modifying the barrier properties of the SC to enhance the drug penetration (and absorption) through the skin can be categorized as; (1) chemical and (2) physical methods of enhancement.
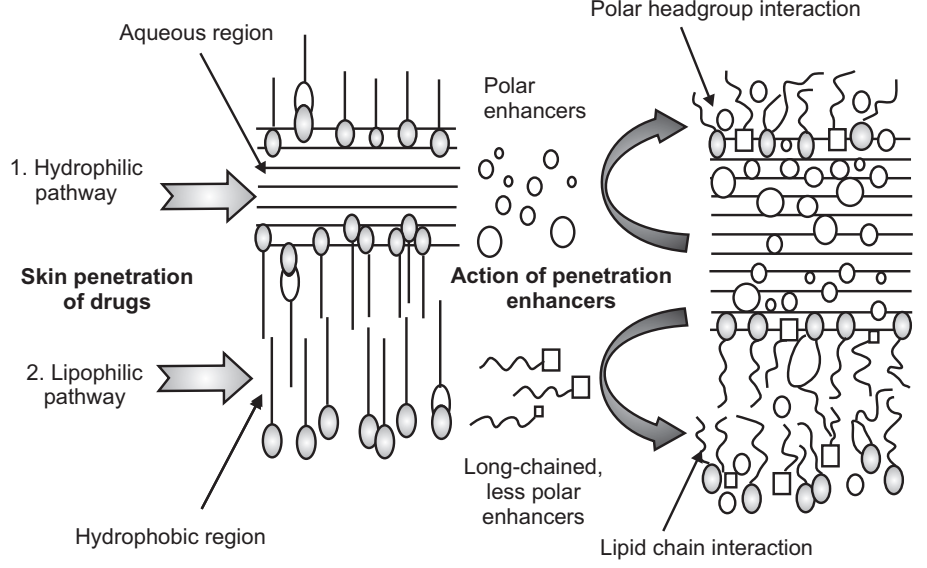
Chemical Enhancers
Chemicals that promote the penetration of topically applied drugs are commonly referred to as accelerants, absorption promoters, or penetration enhancers. Chemical enhancers act by:
- Increasing (and optimizing) the thermodynamic activity of the drug when functioning as a co-solvent.
- Increasing the partition coefficient of the drug to promote its release from the vehicle into the skin. Conditioning the SC to promote drug diffusion promoting penetration and establishing drug reservoir in the SC.
- Some of the more desirable properties for penetration enhancers acting within the skin have been given as: They should be non-toxic, non-irritating, and non-allergenic.
- They should ideally work rapidly and the activity and duration of the effect should be both predictable and reproducible.
- They should have no pharmacological activity within the body, i.e. should not bind to receptor sites.
- The penetration enhancers should work unidirectionally, i.e. should allow therapeutic agents into the body while preventing the loss of endogenous material from the body.
- When removed from the skin, barrier properties should return both rapidly and fully.
- The penetration enhancers should be appropriate for formulation into diverse topical preparations and thus, should be compatible with both excipients and drugs.
- They should be cosmetically acceptable with an appropriate skin “feel” some of the most widely studied permeation enhancers are sulphoxide (DMSO), fatty acids (oleic acid), alcohol (methanol), glycol (propylene glycol), and surfactant (anionic surfactant), azone (lauracapran), etc.
Physical Enhancers
Iontophoresis and ultrasound (also known as phonophoresis or sonophoresis) techniques are examples of physical means of enhancement that have been used for enhancing percutaneous penetration (and absorption) of various therapeutic agents.
PSAs
PSAs are the material that adheres to a substrate, in this case, skin, by application of light force and leaves no residue when removed. They form interatomic and intermolecular attractive forces at the interface, provided that intimate contact is formed. To obtain this degree of contact, the material must be able to deform under slight pressure, giving rise to the term “pressure-sensitive”. Adhesion involves a liquid-like flow, resulting in wetting of the skin surface upon the application of pressure, and, when the pressure is removed, the adhesive sets in that state. A PSA wets and spreads onto the skin when its surface energy is less than that of the skin. After the initial adhesion, the PSA/skin bond can be built by stronger interactions (e.g., hydrogen bonding), which will depend on skin characteristics and other parameters.
Widely used PSA polymers in TDDS are polyisobutylene-based adhesives, acrylics, silicone-based PSAs, hydrocarbon resin, etc. The PSA can be located around the edge of the TDDS or be laminated as a continuous adhesive layer on the TDDS surface. The PSA should be compatible with the drug and excipients, as their presence can modify the mechanical characteristics of the PSA and the drug delivery rate.
Backing Laminates
Backings are chosen for appearance, flexibility, and need for occlusion; hence, while designing a backing layer, the consideration of chemical resistance of the material is most important. Excipient compatibility should also be considered because the prolonged contact between the backing layer and the excipients may cause the additives to leach out of the backing layer or may lead to diffusion of excipients, drugs, or penetration enhancers through the layer. The most comfortable backing will be the one that exhibits the lowest modulus or high flexibility, good oxygen transmission, and a high moisture vapor transmission rate. Examples of backing materials are vinyl, polyethylene, polyester films, aluminum, and polyolefin films.
Release Liner
During storage, the patch is covered by a protective liner that is removed and discarded before the application of the patch to the skin. Because the liner is in intimate contact with the TDDS, the liner should be chemically inert. Typically, a release liner is composed of a base layer that may be non-occlusive (e.g. paper fabric) or occlusive (e.g. polyethylene, polyvinyl chloride) and a release coating layer made up of silicon or Teflon. Other materials used for TDDS release liner are polyester foil and metalized laminates.
Other Excipients Like Plasticizers and Solvents
Various solvents such as; chloroform, methanol, acetone, isopropanol, and dichloromethane are used to prepare drug reservoirs. In addition, plasticizers such as; dibutyl phthalate, triethyl citrate, polyethylene glycol, and propylene glycol are added to provide plasticity to the transdermal patch.
Various Approaches to Transdermal Devices
- Membrane permeation controlled TDDS
- Adhesive dispersion type TDDS
- Polymer matrix diffusion controlled TDDS
- Micro reservoir type TDDS
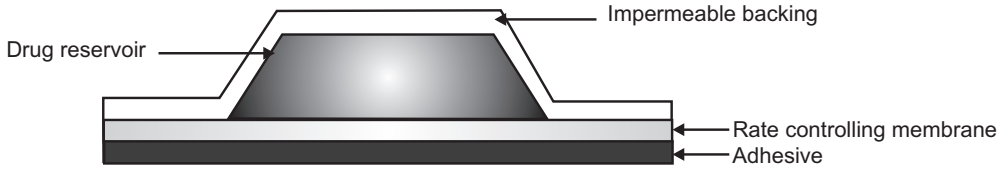
Membrane Permeation Controlled Transdermal Drug Delivery System
- In this type of TDDS, the drug reservoir is sandwiched between drug impermeable backing membrane and rate-controlling membrane.
- The drug releases only through the rate-controlling membrane. It can be microporous or non-porous.
- The drug can be in the form of a solution, suspension, gel, or dispersion in the polymer matrix in the reservoir compartment.
- The release rate of the drug from this type of system can be controlled by varying polymer composition, permeability coefficient, or thickness of the rate-controlling membrane. On the outer surface of the polymeric membrane, a thin layer of adhesive polymer is applied.
- A constant release rate of the drug.
The intrinsic rate of drug release is given by:
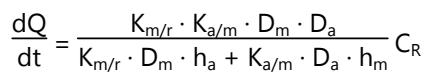
Where,
- dQ / dt = Rate of drug diffusion
- CR = Concentration of drug in the reservoir
- Ka/m = Partition coefficient of drug from membrane to adhesive
- Km/r = Partition coefficient of drug from the reservoir to membrane
- Da = Diffusion coefficient in the adhesive layer
- Dm = Diffusion coefficient in membrane
- ha = Thickness in the adhesive layer
- hm = Thickness of membrane
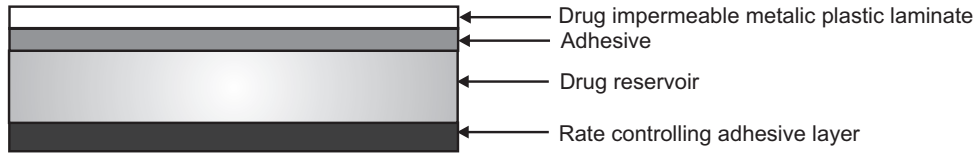
Adhesive Dispersion Type Transdermal Drug Delivery System
- In this type, the drug reservoir is prepared by directly dispersing the drug in an adhesive polymer.
- Then this medicated adhesive polymer is spread over a flat sheet of drug impermeable backing membrane.
- The drug reservoir layer is then covered by a non-medicated rate-controlling polymer of constant thickness to produce an adhesive diffusion controlling DDS.
- The rate of drug release is given as:
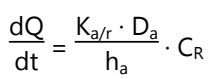
Polymer Matrix Diffusion Controlled Transdermal Drug Delivery System
- Drug reservoir is prepared by dispersing the drug homogeneously in a hydrophilic and lipophilic polymer matrix.
- The resultant medicated polymer is then molded on a medicated disc of defined surface area and thickness.
- The drug reservoir can also be formed by dissolving the drug and polymer in a common solvent and then evaporating of solvent at an elevated temperature.
- This drug reservoir containing polymer disc is then pasted over a base plate containing a drug impermeable backing membrane.
- The rate of drug release is given by:
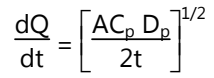
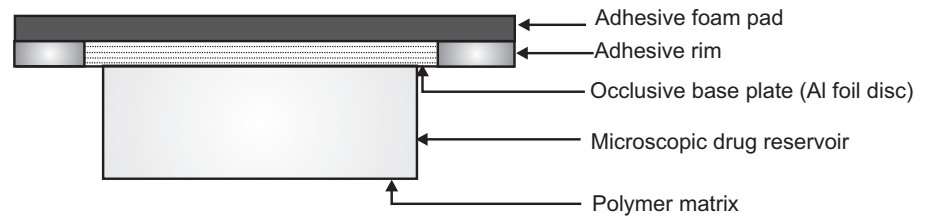
Micro Reservoir Type Transdermal Drug Delivery System
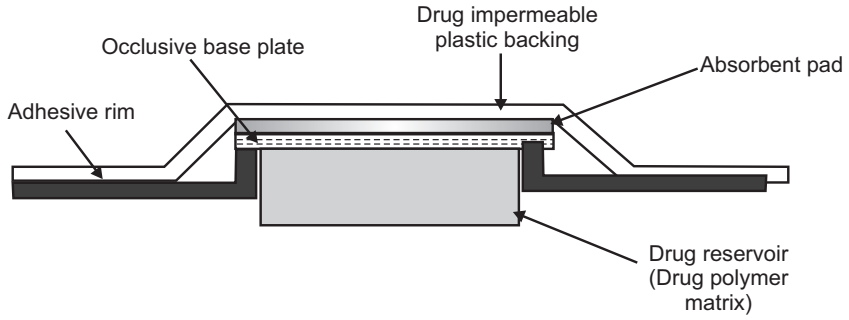
A combination of reservoir and matrix diffusion type drug delivery system.
- A drug reservoir is formed by first suspending the solid drug in an aqueous solution of water-soluble polymer. This drug suspension is homogeneously dispersed in a lipophilic polymer by a high-energy dispersion technique.
- This forms the microscopic spores of drug reservoirs which are supported over an occlusive pad and are thermodynamically unstable.
- Stabilization by cross-linking the polymer chain insitu using a cross-linking agent.
- It can be further coated with a layer of biocompatible polymer to improve the drug release.
Make sure you also check our other amazing Article on : Buccal Delivery System
