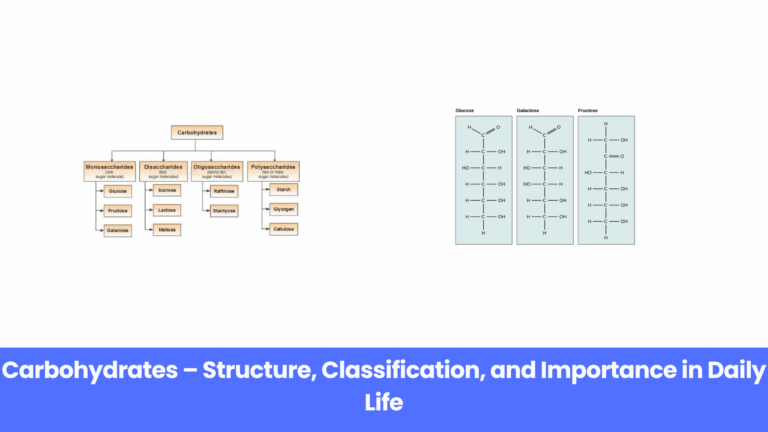
Carbohydrates are one of the most important nutrients that support life on earth. From the food we…
Actress Tamannaah Bhatia recently faced an offensive remark from a journalist during a promotional…
In a story that has gone viral worldwide, a man accidentally became the world’s first…
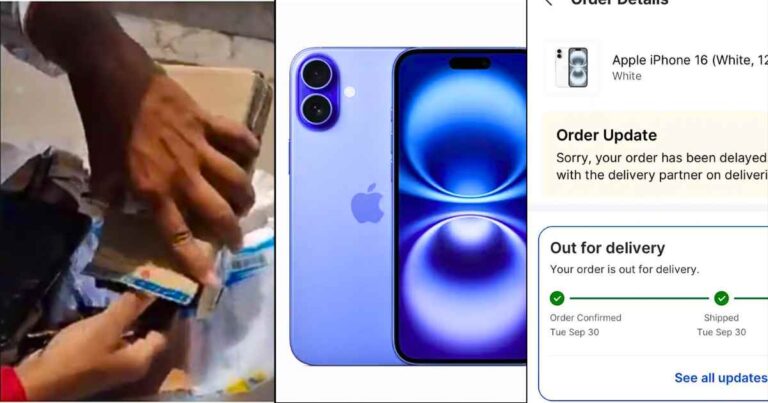
A shocking online shopping incident has gone viral in India. During Flipkart’s Big Billion Days…
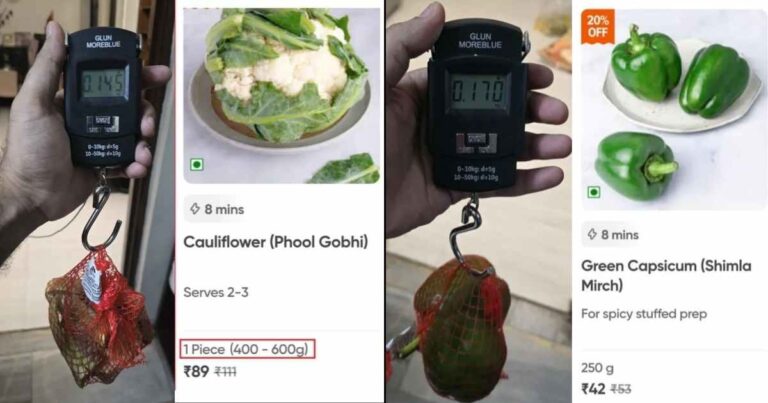
Swiggy Instamart, one of India’s leading online grocery delivery platforms, is facing backlash after…
A video featuring Australian surfer and influencer Ellie Jean Coffey has gone viral, showing her on…
The followers of Vrindavan’s well-known spiritual leader, Premanand Maharaj, have been deeply…
A shocking incident inside a 2nd AC coach of an Indian train has taken the internet by storm. A…
A man from India has taken social media by storm by celebrating his divorce in a bold and unique…
A fan’s unusual request to Shah Rukh Khan (SRK) recently went viral, thanks to the witty reply from…
A Dubai cafe has made headlines by selling a cup of tea worth Rs 1 lakh. The “Gold Karak” tea…
A shocking incident unfolded in a Bihar train when a female government school teacher was caught…
A traveler flying with IndiGo from Chandigarh to Delhi recently encountered a surprising problem at…
Bollywood actor Saif Ali Khan recently revealed a shocking incident where he was attacked by a man…
In a shocking incident, 21-year-old actor Priyanshu, who had worked alongside Bollywood superstar…