Molecular formula: C7H6O2
Molecular Weight: 122.1
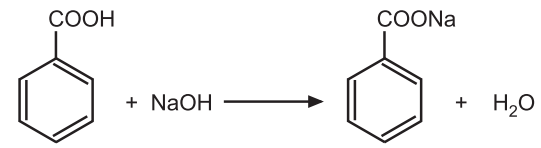
Assay: Weigh accurately about 1.0 g and dissolve in 15 mL of warm ethanol (95%) previously neutralized to phenolphthalein solution. Add 20 mL of water and titrate with 0.5 M sodium hydroxide using phenolphthalein solution as an indicator.
Reaction:
Factor Calculation:
1 Mole of NaOH ≅ 1 Mole of benzoic acid
1000 mL of 1M NaOH ≅ 122.12 g of C7H6O2
1 mL of 0.5M NaOH ≅ 0.06106 g of C7H6O2
Make sure you also check our other amazing Article on : Assay Ammonium Chloride
