Drug recall refers to the action of removing or withdrawing a batch of product from distribution or use, to be returned to the manufacturer. This action is generally done in cases where deficiencies are discovered in the safety, quality, or efficacy of drugs. It is important to note that product recall does not include the normal removal of products that have passed their expiry period.
The Organization of Pharmaceutical Producers of India (OPPI) defines recall as, “An action taken to resolve a problem with therapeutic goods for which there are established deficiencies in quality, efficacy or safety.”
According to the CDSCO (Central Drugs Standard Control Organization), quality defects may include drugs that are not of standard quality, spurious or adulterated drugs. Safety and efficacy defects include serious adverse drug reactions and death. Drugs manufactured despite being prohibited under the provisions of the Drugs and Cosmetics Act, and products manufactured under canceled or suspended license may also be recalled from the market.

Types of Drug Recall:
Table of Contents
Product recalls may be of two types:
(a) Voluntary recall: This refers to situations when the manufacturer decides on their initiative to recall products where the safety, efficacy, and quality of a batch is in question. For example:
1. Batch does not comply with regulatory requirements during stability study done in the post-marketing phase.
2. An in-house investigation reveals a failure (cross-contamination or mix-up) that may harm the quality of a batch of products that have already been released for distribution.
3. Market complaint investigations show that the entire batch of distributed products is defective.
4. Visual inspection of retained samples shows evidence of deterioration that has an impact on product quality.
5. Pharmacovigilance reports indicate a serious safety risk to those taking the medication.
(b) Statutory recall: These are recalls mandated by drug regulatory bodies at Central or State levels for one of the following reasons:
1. The product violates the law – for example, it is not of standard quality.
2. The formulation contains banned drugs.
3. The labeling of the product or promotional material violates the law.
4. Product is found to contravene provisions of Schedule J (it claims to cure a disease/disorder that no drug can claim to do)
Regulatory bodies encourage manufacturers to implement a product recall voluntarily if they discover that any of their products are defective. However, in cases where the regulatory body finds evidence of seriously defective or dangerous products, it may suggest or order a product recall by sending a notice to the concerned company. In case of non-compliance by the company to such an order, legal action may be initiated against the manufacturer.
In cases where the product recall is ordered by the regulatory body, the concerned company may choose to contest the notice. However, it is important to do a complete review of the actual situation and take necessary legal advice before proceeding in this direction.
A Recall is Different from Withdrawal:
A drug recall is a removal from the market due to quality, safety, or efficacy issues. Drug withdrawal, on the other hand, is a change in the approval status of the drug by the concerned regulatory authorities. If the nature and frequency of adverse drug events are such that the risks of a drug far outweigh its benefits, the regulatory body may decide to withdraw approval for the manufacturing of such a product, and the manufacturer is informed to stop producing it.
Reasons for Product Recall:
Product recall may have to be carried out if:
- Potentially dangerous/serious product quality issues have come to light through complaints or other means.
- Mandatory regulations have been violated and come to the notice of regulatory agencies, who then order a recall.
- Field monitoring studies/other reports show evidence of tampering with the product.
- New information that comes to light after distribution of a product indicates it is unsafe or ineffective or dangerous.
Most common reasons for drug recalls:
- cGMP violations.
- Microbial contamination in non-sterile products.
- Failing dissolution test requirements.
- Degradation products/impurities.
- Lack of efficacy.
- Labeling errors (declared strength).
- Lack of assurance of sterility.
- Misbranded drug (therapeutic claims that are unapproved in promotional literature).
- Lack of drug stability.
- Incorrect outer packaging (correct label on a product, packed in incorrect carton).
Classification of Recall:
The CDSCO classifies recalls into three categories:
Class I is the situation in which there is a reasonable probability that the use of, or exposure to, a defective product will cause serious adverse health consequences or death and as well as drugs banned under 26A of Drugs and Cosmetics Act 1940. Recalls under this class must be executed to the level of distributor/wholesaler, retailer, and consumer. Public announcements shall be made using print and electronic media. Timeline for recall ranges from within 24 hours up to a maximum of 72 hours – stopping the sale/distribution must be enforced within 24 hours; physical recall must be completed within 72 hours.
Class II is a situation in which the use of, or exposure to, a defective product may cause temporary adverse health consequences or where the probability of serious adverse health consequences is remote. These recalls are implemented at the level of distributor/wholesaler and retailer. The time limit for the recall is a maximum of 10 days.
Class III is a situation in which the use of, or exposure to, a defective product is not likely to cause any adverse health consequences. Here, recall is executed until the wholesaler level, and a time limit of up to 30 days is permitted.
Banned drugs for which license is canceled or suspended, if found to be in the market, shall have to be recalled, being treated as Class I recall.
Mock Recall:
As per CDSCO guidelines, companies must carry out a mock recall for at least one batch of any one of their products after being dispatched. It is best to choose a product where maximum distributors are involved. This helps to evaluate how effective the arrangements are to execute the product recall. Such a mock recall must also be carried out in the event of a change in the marketing/distribution partner. Records of mock recalls must be maintained by the QA department.
USFDA Classification of product recalls
Class I: Situations that involve a threat to life. FDA orders a consumer recall, a 100 % effectiveness check, and requires public announcements to be made about the hazards of the product.
Class II: Situations that are potentially hazardous but not life-threatening. FDA orders recall to retail outlets but a 100% effectiveness check is not mandatory. Depending on the reason for the recall, a press release may or may not be necessary.
Class III: Situations that do not pose a serious hazard. The recall is restricted to wholesale outlets. Effectiveness checks are not necessary, no press release is required either.
Product recall guidelines – worldwide
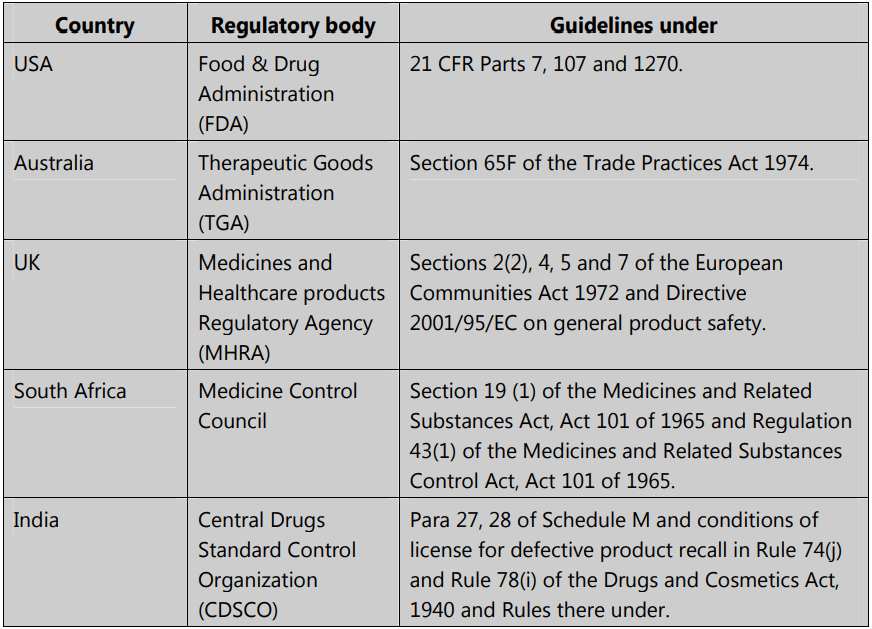
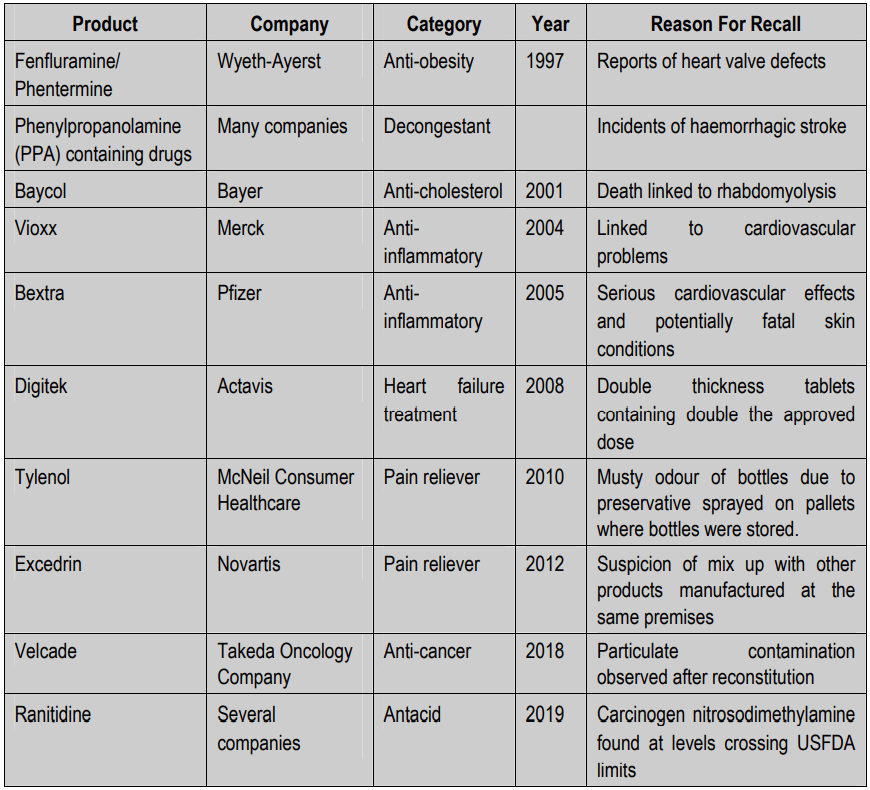
Major drug recalls
Product Recall System:
Every company is required to have a product recall system in place to effect a prompt and effective recall in case of serious complaints regarding defective products. As per cGMP guidelines, product recall activities must be coordinated and executed by an authorized person who is independent of the marketing and sales function of the company. The recall approving authority is generally the Head of the Company (President/Proprietor/Managing Director).
Recall strategy details must include information regarding:
1. Authorized person who will initiate the recall.
2. Nature of communication that will be used to initiate recall (telephone, email, letters, etc.).
3. Depth of the recall to be instituted (recall from distributor/wholesaler/hospital/ retailer/general public).
4. Manner of receiving, segregating, and secure storage of the recalled product.
5. Reconciliation reports being prepared, at what frequency.
6. Verification of success of recall and report submission to regulatory authorities.
7. Steps to be taken to avoid re-occurrence of the same issue with the product.
8. Dealing with the recalled product – reworking or destruction as may be appropriate.
Make sure you also check our other amazing Article on : Out of Specification Results