Techniques or methods used for the Evaluation of Antimicrobial Agents and Disinfectants are:
- Tube dilution and agar plate method.
- Filter paper and cup plate method.
- Ditch-plate method.
- Phenol coefficient method.
- Kelsey Sykes method.
Tube dilution and agar plate method
Table of Contents
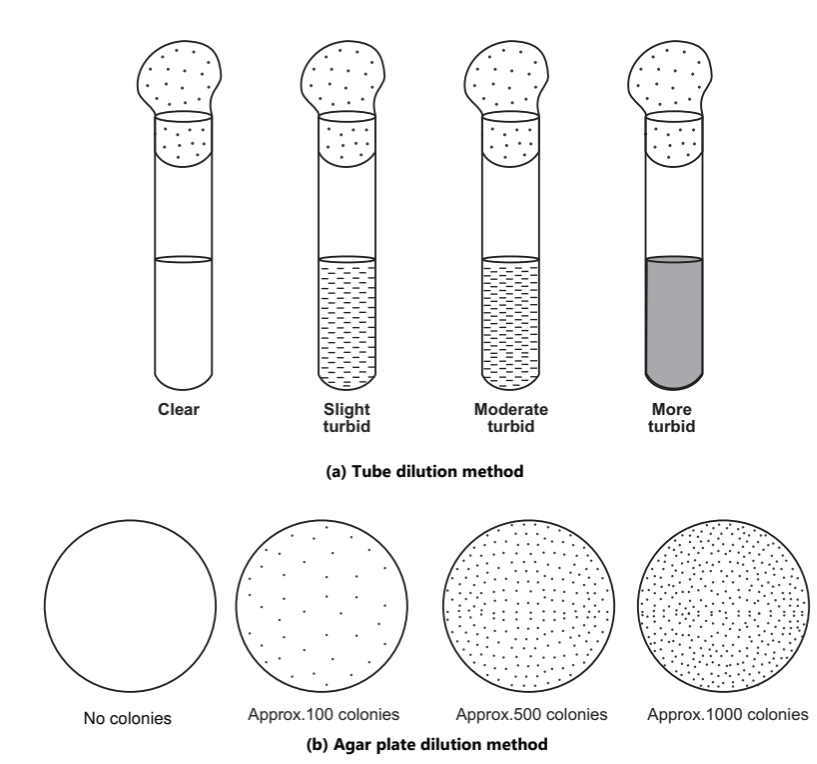
The chemical agent is incorporated into nutrient broth or agar medium and inoculated with the test microorganisms. These tubes are incubated at 30 to 35 oC for 2 to 3 days and then the results in the form of turbidity or colonies are observed. The results are recorded and the activity of the given disinfectant is compared as shown in Fig.1.
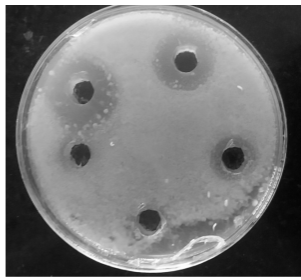
Filter paper, cup-plate, and cylinder plate method
In these methods, the agar is melted, cooled at 45 oC, inoculated with the test microorganisms, and poured into a sterile Petri plate. In the cup-plate method, when the inoculated agar has solidified, holes about 9 mm in diameter are cut in the medium with a sterile cork borer. The antimicrobial agent is directly placed in the holes (Fig.2). In the filter paper and cylinder plate method, the antimicrobial agent is applied to the surface of the solidified, inoculated agar by using a filter paper disc and cylinder respectively. The zone of inhibition is observed after incubation at 30 to 35 oC for 2 to 3 days. The diameter of the zone of inhibition indicates the relative activities of different antimicrobial substances against the test microorganisms.
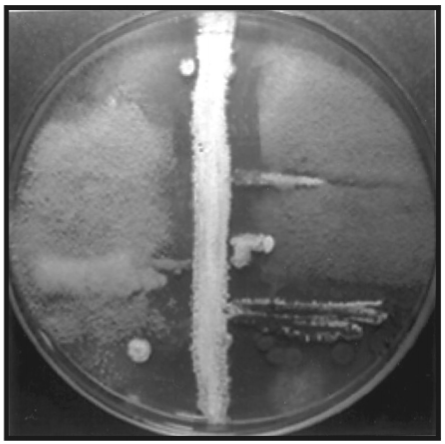
Ditch-plate method
A solution of the antimicrobial substance is carefully run into the ditch which is prepared in an agar plate. A loopful of each test microorganism is then streaked outwards from the ditch on the agar surface. Microorganisms resistant to the antimicrobial grow right up to the ditch whereas susceptible microorganisms show a zone of inhibition adjacent to the ditch or center of the plate. The width of the inhibition zone (Fig.3) indicates the relative activity of the antimicrobial substance against the various test microorganisms.
Phenol coefficient method
In the phenol coefficient method, a test chemical is rated for its microbicidal property concerning phenol under identical conditions. In this test, similar quantities of
microorganisms are added to rising dilutions of phenol and of the disinfectant to be tested. In the U.K. the organism used is Salmonella typhi and in the U.S.A., Salmonella typhi, Staphylococcus aureus, and Pseudomonas aeruginosa are used.
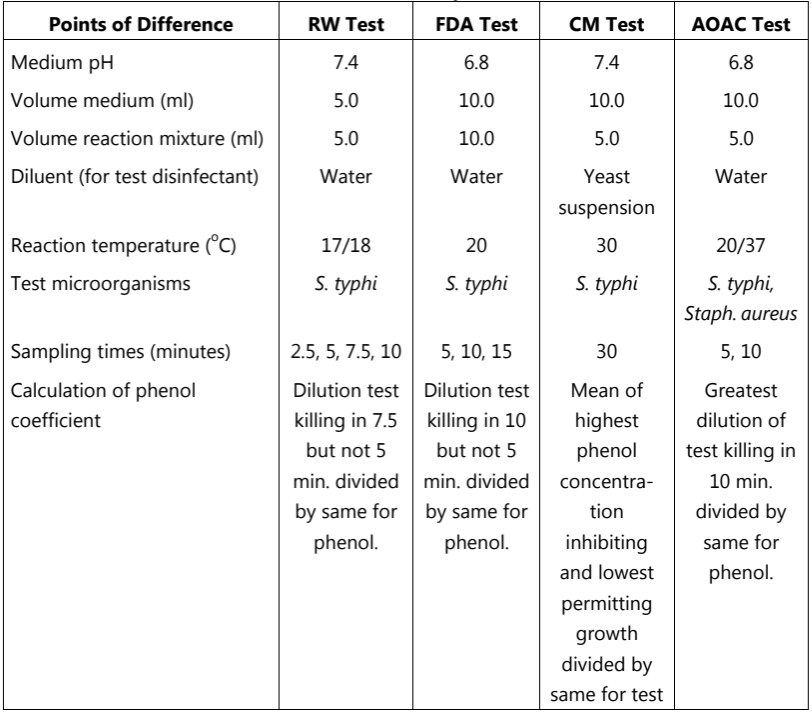
The phenol coefficient test includes:
- Rideal – Walker test (RW test)
- Chick–Martin test (CM test)
- United States Food and Drug Administration test (FDA test).
- The US Association of Official Agricultural Chemists test (AOAC test).
The phenol coefficient test is suitable for testing disinfectants miscible with water and which exert their antimicrobial action like that of phenol.
The phenol coefficient of test disinfectants may be calculated by Rideal – Walker test that uses Rideal-Walker broth and Salmonella typhi as the sensitive microorganism.
Different dilutions of the test disinfectant and phenol are prepared and 5 ml of each dilution is inoculated with 0.5 ml of the 24-hour broth culture of the organisms. All tubes (disinfectant organisms and phenol organisms) are placed in a 17.5 oC water bath. Subcultures of each reaction mixture are taken and transferred to 5 ml sterile broth after 2.5, 5, 7.5, and 10 min.
The broth tubes are incubated at 37 oC for 48 to 72 hours and are examined for the presence or absence of growth. A typical result is shown in Table.1. The Rideal-Walker coefficient of the test disinfectant is then calculated (as per results shown in Table.1) as follows:
Table.1: Determination of Rideal-Walker coefficient
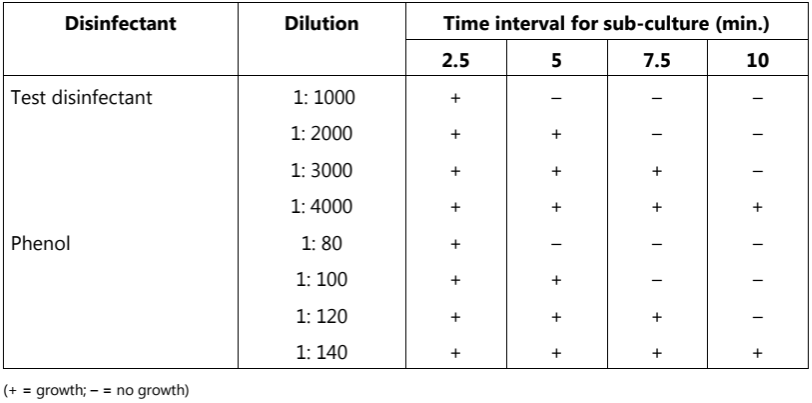

If the phenol coefficient or Rideal-Walkar coefficient of the given test disinfectant is 1, it means that the disinfectant has the same effectiveness as phenol. The phenol coefficient of a test disinfectant less than 1 means it is less effective and more than 1 means it is more effective as compared to phenol. If the phenol coefficient of the test disinfectant is 20 it means that the disinfectant is 20 times more active than phenol. The other main phenol coefficient tests are given in Table.2.
Table.2: Differences between phenol coefficient test
Advantages of the phenol coefficient test:
- They are inexpensive and can be performed quickly.
- They give reproducible results in the hands of experienced workers.
- They are valuable to eliminate useless products and supply standards for crude preparations.
Disadvantages of the phenol coefficient test:
- Choice of the test organism: In most tests, only one organism is used i.e. Salmonella typhi. Results for this organism give only limited information on how the disinfectant will behave against other organisms. The use of Salmonella typhi is undoubted because phenol coefficient tests originated in 1903 when typhoid organisms were a much greater menace than it is in the present times.
- Phenol coefficient tests compare the activity of bacteriocides at only one concentration with a fixed death time and reaction temperature.
- Most phenol coefficient tests do not indicate the activity of disinfectants in the presence of organic matter.
- These tests do not give any information related to tissue toxicity.
- Sampling errors are large in phenol coefficient tests.
- Phenol coefficient tests do not give any indication of the effects of dilution on the activity of the disinfectant and are used to evaluate phenolic disinfectants only.
Kelsey-Sykes method (1969)
In this method, several test bacteria such as Staphylococcus aureus, Proteus vulgaris, Escherichia coli, and Pseudomonas aeruginosa are used. This test can be carried out in clear or dirty conditions. In both cases, the final concentration of bacterial cells should be about 109/ml. Clean conditions are simulated by using broth as the suspending fluid and dirty conditions by the use of a yeast suspension or inactivated horse serum as the suspending fluid. The dilutions of the disinfectant are made in hard water. The basic procedure of the Kelsey-Sykes method is outlined in Table.3.
Table.3: Procedure of Kelsey-Sykes test
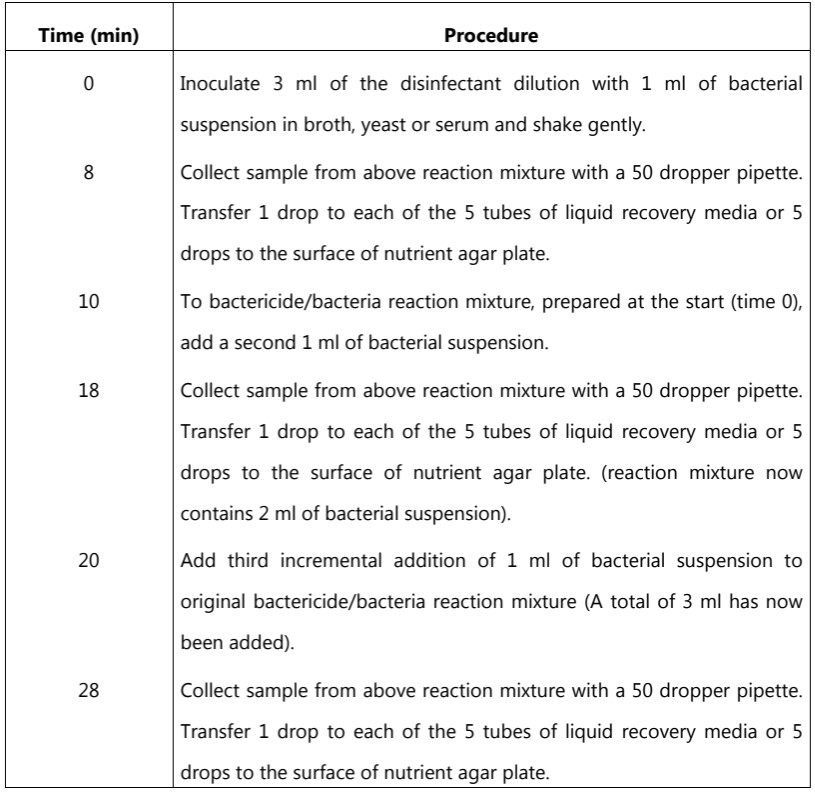
The samples taken at 8, 18, and 28 minutes are then incubated at 30 to 32 oC and the number of tubes showing growth or the number of colonies/drops from surface plate culture is recorded.
Interpretation of result: A disinfectant is satisfactory for use at the initial concentration if:
(a) no growth occurs in 2 or more of the 5 tubes of the 18 minutes samples i.e. subcultures taken after the second incremental addition of bacteria; or
(b) there are not more than 5 colonies from the 5 drops on the agar plate.
Make sure you also check our other amazing Article on: Factors Affecting Disinfectant Action