SCALE-UP LIQUID ORALS
Table of Contents
1. The physical form of a drug product that is pourable displays Newtonian or pseudoplastic flow behavior and conforms to its container at room temperature.
2. Liquid dosage forms may be dispersed systems or solutions.
3. In dispersed systems there are two or more phases, where one phase is distributed in another.
4. A solution refers to two or more substances mixed homogeneously.
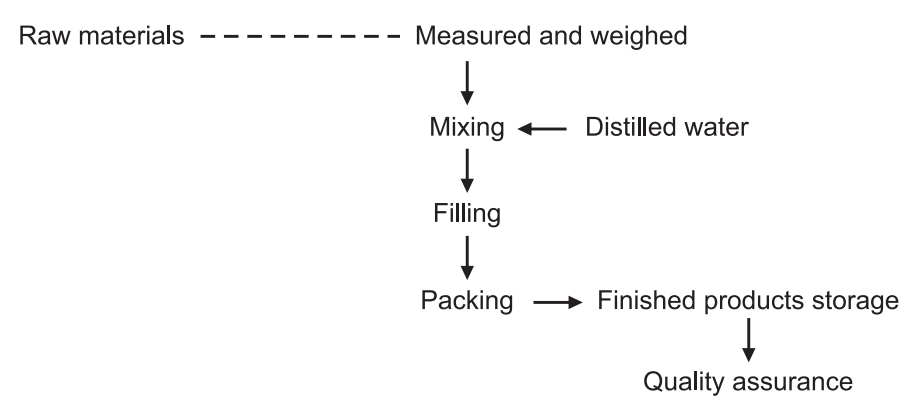
Steps in Liquid Manufacturing Process:
1. Planning of material requirements.
2. Liquid preparation.
3. Filling and packing.
4. Quality assurance.
Critical Aspects of Liquid Manufacturing:
Formulation Aspects of Suspensions
| Sr.No. | Purpose | Agent |
| 1. | Facilitating the connection between API and vehicle. | Wetting agents. |
| 2. | Protecting the API. | Buffering systems, polymers, antioxidants. |
| 3. | Maintaining the suspension appearance. | Colorings, suspending agent, flocculating agent. |
| 4. | Asking the unpleasant taste/smell. | Sweeteners, flavorings. |
Formulation Aspects of Emulsions
| Sr.No. | Purpose | Agent |
| 1. | Particle size | Solid particles, droplet particles |
| 2. | Protecting the API | Buffering systems, antioxidants, polymers |
| 3. | Maintaining the appearance | Colorings, emulsifying agents, penetration enhancers, gelling agents |
| 4. | Taste/smell masking | Sweeteners, flavorings |
Formulation Aspects of Solutions
| Sr.No. | Purpose | Agent |
| 1. | Protecting the API. | Buffers, antioxidants, preservatives. |
| 2. | Maintaining the appearance. | Colorings, stabilizers, co-solvents |
| 3. | Antimicrobial. | Preservatives. |
| 4. | Taste/smell masking | Sweeteners, flavorings |
Types of Equipment Used:
1. Mixer
2. Homogenizer
3. Filtration assembly
4. Bottling assembly
General Flow Chart for Manufacturing Liquid Orals
Quality Assurance:
- Dissolution of drugs in solution
- The potency of drugs in suspension
- Temperature uniformity in emulsions
- Microbiological control
- Product uniformity
- Final volume
- Stability

SEMI-SOLID DOSAGE FORMS
- In general, semisolid dosage forms are complex formulations having complex structural elements.
- Often, they are composed of two phases (oil and water), one of which is a continuous (external) phase, and the other of which is a dispersed (internal) phase.
- The active ingredient is often dissolved in one phase, although occasionally the drug is not fully soluble in the system and is dispersed in one or both phases, thus creating a three-phase system.
Parameters:
1. For a true solution, the order in which solutes are added to the solvent is usually unimportant.
2. The same cannot be said for dispersed formulations, however, because dispersed matter can distribute differently depending on to which phase a particulate substance is added.
3. In a typical manufacturing process, the critical points are generally the initial separation of a one-phase system into two phases and the point at which the active ingredient is added.
4. This is particularly important for solutes added to the formulation at a concentration near or exceeding that of their solubility at any temperature to which the product may be exposed.
5. Variations in the manufacturing procedure that occur after either of these events are likely to be critical to the characteristics of the finished product.
6. This is especially true of any process intended to increase the degree of dispersion through reducing droplet or particle size (for example, homogenization).
7. Aging of the finished bulk formulation before packaging is critical and should be specifically addressed in process validation studies.
Make sure you also check our other amazing Article on : Pilot Plant Scale-Up Techniques For Capsules