Manufacturing Facilities for Ophthalmic Preparations: Environmentally controlled areas are used for manufacture. Class 100 space is the area where not more than 100 particles are present per cubic foot of air of a diameter of 0.5 micrometers or larger. Similarly, class 1,00,000 space is defined. Class 100 space is required for filling and capping operations. For this purpose HEPA, filtered laminar airflow sources are used. Class 1,00,000 space is required for the storage of raw materials, finished products.
Walls, ceilings, and floors should be constructed of materials that are hard, non-chipping, or non-flaking, smooth, and unaffected by surface cleaning agents and disinfectants.
All lights and windows should be flush mounted in walls and ceilings for ease of cleaning and disinfection. Ultra Violet lamps may be provided in recessed, flush-mounted fixtures to maintain surface disinfection.
Separate entrances for personnel and equipment should be provided through specially designed airlocks that are maintained at a negative pressure relative to the aseptic manufacturing area and a positive pressure relative to an environmentally controlled area.
Equipment should be designed for simplicity of operation and should be constructed for ease of disassembly, cleaning, and sterilization.
Personnel must be trained in the proper mode of gowning with sterile, non-shedding garments, and also in the proper techniques and conduct for aseptic manufacturing. A cool working environment should be maintained, with relative humidities controlled to between 40 and 60% RH.
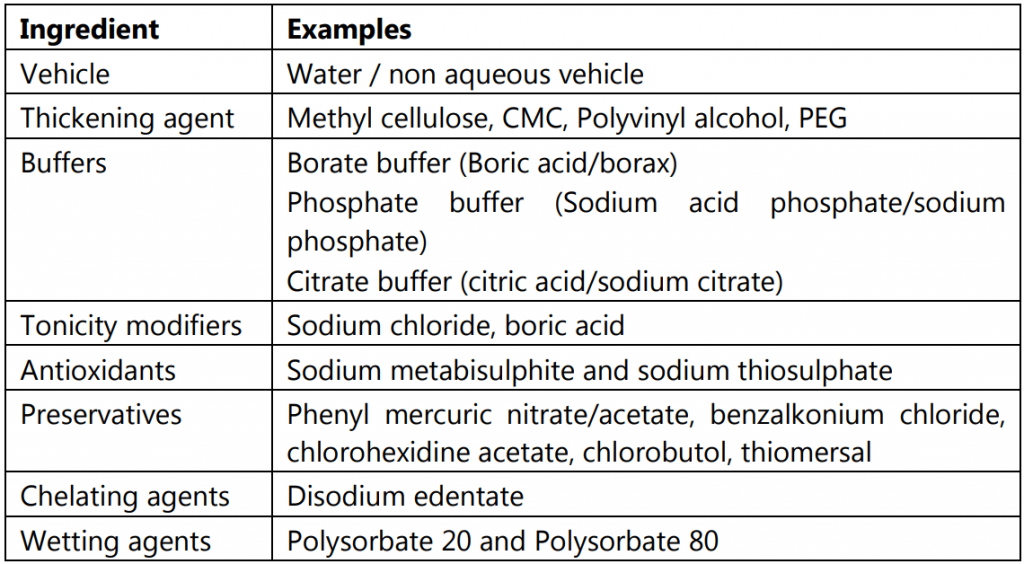
Raw Materials: All raw materials used in the compounding of ophthalmic pharmaceutical products must be of the highest quality available. Complete raw material specifications for each component must be established and verified for each lot purchased.
Equipments: All tanks, valves, pumps, and piping must be of the best available grade of corrosion-resistant stainless steel. All product-contact surfaces should be finished either mechanically or by electro-polishing to provide a surface as free as possible from scratches or defects that could cause corrosion. Care should be taken in the design of such equipment to provide adequate means of cleaning and sanitization.
Types:
1. Ophthalmic drops
2. Ophthalmic suspensions
3. Ophthalmic lotions
4. Ophthalmic ointments
5. Ophthalmic inserts
Ophthalmic Drops
Table of Contents
Ophthalmic drops (solutions) are sterile aqueous/oily solutions, essentially free from foreign particles, suitably compounded, and packaged for instillation into the eye. The ophthalmic drops must be protected from contamination during use and must be used within 2 weeks after the first opening of the container. For this reason, they should be prescribed in small amounts i.e. 5 to 10 ml.

Requirements:
(i) Sterility: They should be sterile.
(ii) Tonicity: They should be isotonic with lachrymal secretions
(iii) Clarity: They should be free from foreign particles, fibers, and filaments.
(iv) pH: They should have almost neutral pH.
(v) Preservation: They should be preserved with a suitable preservative.
(vi) Buffer action: They should have buffering capacity.
(vii) Storage: They should remain stable during their storage.
(viii) Package: They should be packaged in dropper containers so that they must be easy to instill into the eyes.
Types of drugs supplied in the eye drops form are:
Antiseptics, Anesthetics, Anti-inflammatories, Mydriatics, Miotics, and Diagnostic aids.
Formulation:
Method of Preparation of Eye Drops:
(i) Dissolution: Dissolve the drug and excipients (all or part) in the water (all or part).
(ii) Sterilisation: Sterilise the solution by heat or by filtration through sterile depth or membrane filter media into a sterile receiver.
(iii) Addition of excipients: If the procedure is incomplete, the sterile solution is then mixed with the additional required sterile excipients such as sterile viscosity imparting agents, preservatives, etc.
(iv) Make up the volume: Finally, the volume is made up of sterile water.
(v) Filling and packaging: The solution after filtration is filled into borosilicate glass containers or plastic containers in aseptic condition. The containers are immediately closed using suitable closures.
(vi) Sterilization: A few drugs are dissolved in simple aqueous vehicles that are stable to normal autoclaving temperatures and times (121o C for 20-30 minutes). Such drug products must be packaged in glass or heat resistant packages which are terminally sterilized by autoclaving.
Most ophthalmic products are not stable to heat either physically or chemically. Because of the product sensitivities, most ophthalmic products are aseptically manufactured and filled into previously sterilized containers in aseptic environments using aseptic filling and capping techniques. Such products are not terminally sterilized.
(vii) Labeling: The label should contain the following directions :
“Not for injection.”
“If irritation persists, discontinue the use and consult a physician.”
“Discard the preparation, if any particles are seen.”
“Don’t touch the tip of the dropper to any surface.”
Ophthalmic Suspensions:
Ophthalmic suspensions are sterile biphasic liquid dosage forms containing solid particles dispersed in a liquid vehicle intended for application to the eye. These are not commonly used as compared to eye drops. These are preferred to formulate under the following conditions:
(i) When the drug is insoluble in the desired vehicle.
(ii) When the drug is unstable in solution form.
(iii) When sustained action is desired.

Requirements:
(i) Sterility: They should be sterile.
(ii) Tonicity: They should be isotonic.
(iii) Viscosity: They should possess desired viscosity.
(iv) Buffer action: They should have buffering capacity.
(v) Particle size: The particle size should be non-irritating and non-scratching to the cornea. The drug used is in a microfine form, usually, 95% or more of the particles have a diameter of 10 micrometers or less.
(vi) Distribution of particles: The suspended particles must get distributed uniformly throughout the vehicle.
(vii) Storage: The suspended particles must not agglomerate into larger ones on storage.
(viii) Package: They should be packaged in dropper containers so that they must be easy to instill into the eyes.
(ix) Auxiliary label: Shake well before use.
Examples of drugs used in the form of suspensions are:
(a) Tetracycline hydrochloride
(b) Oxytetracycline HCl and Hydrocortisone acetate
(c) Tobramycin and Dexamethasone
(d) Prednisolone phosphate
Ophthalmic Lotions:
Eye lotions are sterile aqueous liquids used for washing the eyes.
Requirements:
(i) Sterility: They should be sterile.
(ii) Tonicity: They should be isotonic.
(iii) pH: The should have almost neutral pH. Tonicity and pH in eye lotions are more important than in eye drops because of the large volumes administered. Additives such as buffers and isotonic salts are used for this purpose.
(iv) Preservation: These are generally used for a single time and do not contain any preservatives. If they are formulated for multiple uses, these should contain a suitable preservative.
(v) Shelf life: They should be freshly prepared and should not be stored for more than 2 – 3 days.
(vi) Direction to use: These are usually supplied in concentrated form and are required to be diluted with an equal volume of warm water before use.
(vii) Mode of administration: These are usually applied with a clean eye bath or sterile fabric dressing and a large volume of solution is allowed to flow quickly over the eye.
(viii) Use: These are applied in relatively large volumes to remove foreign materials and to relieve irritation.
(ix) Labeling conditions: The label should bear the same directions as that of eye drops.
Examples of drugs used for the preparation of eye lotions are sodium chloride, sodium bicarbonate, boric acid, borax, and zinc sulfate.
Ophthalmic Ointments:
Ophthalmic ointments are sterile semisolid dosage forms meant for administration into the eye.
Requirements of ophthalmic ointment bases:
(i) Base must be non-irritating to the eye.
(ii) Base must permit the diffusion of the medicinal substance throughout the secretions bathing the eye.
(iii) Base must have melting or softening point close to body temperature.
(iv) Base must be able to sterilize and retain its sterility.
Examples of ophthalmic ointment base: The principal semisolid dosage form used in ophthalmology is an anhydrous ointment with a petrolatum base. The most commonly used base is
Yellow soft paraffin: 80 g
Liquid paraffin: 10 g
Wool fat: 10 g
Method of preparation: Melt wool fat, yellow soft paraffin on a water bath. Add liquid paraffin. Filter through coarse filter paper placed in a heated funnel. It is sterilized by the dry heat method (160o C for 2 hours). The drug is added to the ointment base either as a solution or as a finely micronized powder. The drug is then intimately mixed with the base, usually by milling.
White soft paraffin is not used in the preparation of ointment base because it is prepared by bleaching the yellow soft paraffin. Some of the bleaching agents may remain adhered to the base even after careful washing which when used in the eye may lead to irritation. Wool fat is used to ensure satisfactory emulsification of the solution and helps in the absorption of active ingredients. Liquid paraffin is incorporated to reduce the melting pint and viscosity of the base so that it can be easily expelled from the collapsible tube and apply to the eye.
Filling: After preparation, the ophthalmic ointments are filled into a previously sterilized tin or plastic tubes. These tubes are typically small, holding approximately 3.5g of ointment and fitted with narrow gauge tips which permit the extrusion of narrow bands of ointment. The container components and the exterior surface of the final packages are sterilized by heat, ethylene oxide gas, or ionizing radiation.
General Method of Manufacturing Ointments:
(i) All raw materials used must be sterile or if possible ingredients are dissolved in water and are then sterilized by heat, filtration, or radiation.
(ii) The ointment base is sterilized by heat and filtered while molten to remove extraneous foreign particulate matter.
(iii) The molten state of the sterilized base is placed into a sterile steam jacketed kettle to maintain the ointment in a molten state under aseptic conditions.
(iv) Previously sterilized active ingredients and excipients are added aseptically.
(v) While still molten, the entire ointment may be passed through a previously sterilized colloid mill for adequate dispersion of the insoluble components.
Labeling: In addition to the general labeling requirements, the label on the container or the sealed outer package enclosing an eye ointment, should indicate that the contents are sterile provided that the container has not been opened.
Advantage:
The primary advantage of an ophthalmic ointment over an ophthalmic solution is the increased ocular contact time of the drug to increase drug bioavailability.
Disadvantages:
Greasy nature
The blurred vision occurs as the ointment base melts and is spread across the lens.
Examples:
- Atropine sulfate eye ointment
- Hyoscine eye ointment
- Chloramphenicol eye ointment
- Hydrocortisone eye ointment
- Chlortetracycline eye ointment
- Neomycin sulfate eye ointment
- Mercuric oxide eye ointment
- Tetracycline HCl eye ointment
- Gentamicin sulfate eye ointment
Ophthalmic Inserts (Ocusert):
An ophthalmic insert is a dosage form that administers a drug or drugs at programmed rates, at a specific body site, for a prescribed period to provide continuous control of drug therapy and to maintain this control over extended periods.

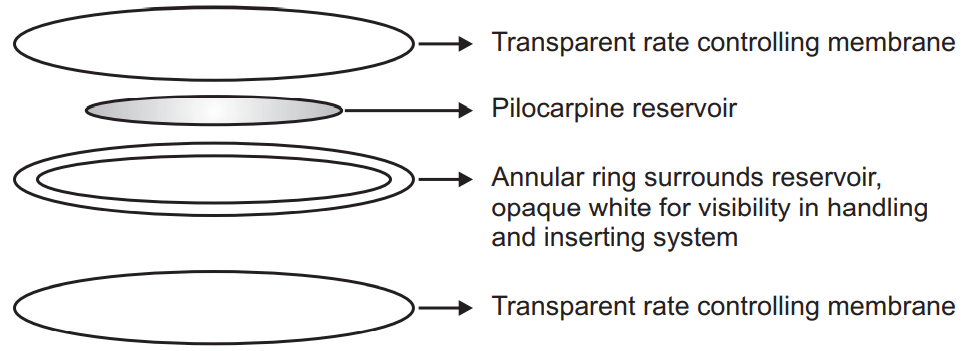
Shape and structure of the Ocusert: The Ocusert is a soft, flat, flexible, sterile, and elliptical device with dimensions of 13.4 x 5.7 x 0.3 mm. The elements of the Pilocarpine Ocusert system are shown in Fig.1. Russert consists of
(a) pilocarpine reservoir in alginate base,
(b) rate-controlling membranes of EVA (ethylene vinyl acetate), and
(c) annular ring.
The platform component for the Pilocarpine Ocusert consists of the EVA copolymer membranes, which serve as the housing. An annular ring of the membrane impregnated with titanium dioxide forms a white border for visibility. The free-base form of pilocarpine is used since it exhibits both hydrophilic and lipophilic characteristics. The drug diffuses through these membranes at a constant rate.
It is designed to be placed in the inferior cul-de-sac between the sclera and the eyelid and to release pilocarpine at a constant rate of 20 or 40 µg per hour around the clock for 7 days. The rate of drug diffusion is controlled by the polymer composition, the membrane thickness, and the solubility of the drug. The devices are sterile and do not contain preservatives.
Advantages:
- The Ocusert exposes a patient to only one-fourth to one-eighth the amount of pilocarpine, compared with drop therapy.
- This could lead to reduced local side effects and toxicity.
- It provides a precisely controlled rate of delivery and therefore around-the-clock control of intraocular pressure, whereas drops used four times a day can permit periods where the intraocular pressure might rise.
- The Ocusert provides for more patient convenience and improved compliance, as the dose needs to be administered only once per week.
- It is designed to provide for the release of medication at predetermined and predictable rates.
- It permits the elimination of frequent dosing by the patient.
- It ensures nighttime medication.
Disadvantages:
- The clinical experience seems to indicate that the Ocusert has a compliance problem of its own (i.e. retention in the eye for the full 7 days).
- The patient must check periodically to see that the unit is still in place, particularly in the morning on rising.
- Replacement of a contaminated unit with a fresh one can increase the price differential of the already expensive Ocusert therapy compared with an inexpensive drop or once-a-day gel therapy.
Make sure you also check our other amazing Article on : Preparation of Sterile Powders