The determinate error may be minimized by using the following methods:
1. Running a blank determination: Errors arising from the introduction of impurities through the reagents and vessels are accomplished by running a blank. Such a procedure involves going through all the analysis, using the same solvent and reagent in the same quantities, but omitting the unknown component. Thus, in making a blank, a sample is omitted; otherwise, the details of the procedure are followed exactly as far as possible.
2. Calibration of apparatus and application of corrections: All instruments, such as burettes, pipettes, weights, measuring flasks, etc. must be properly calibrated and the appropriate corrections must be applied to the original measurements.
3. Running a controlled determination: It consists in carrying out a determination under identical experimental conditions as far as possible upon a quantity of a standard substance, which contains the same weight of the constituent as it contained in the unknown sample.
The weight of the constituent ‘X’ in the unknown sample can then be calculated.
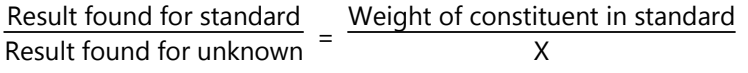
4. Running of parallel determination: Parallel determinations serve as a check in the result of a single determination and indicate only the precision of the analysis. The values obtained in parallel determination should agree well among themselves. These values should not vary by more than three parts per thousand. If larger variations are shown the determination must be repeated until satisfactory concordance is obtained. A good agreement between, duplicate and triplicate determinations does not justify the conclusion that the result is correct, but it merely shows that the accidental errors or variations of the determinate errors are the same in parallel determinations.
5. Standard addition: A known amount of the constituent being determined is added to the sample, which is then analyzed for the total amount of constituent present. The difference between the analytical results for samples with and without the added constituent gives the recovery of the amount of added constituent. If the recovery is satisfactory, the accuracy of the procedure is enhanced. This procedure is especially applied to physicochemical processes, such as polarography and spectrophotometry.
6. Isotopic dilution: It consists in adding a known amount of pure component containing a radioactive isotope to the unknown, now the element so isolated is obtained in pure form usually as a compound. Its activity is determined with the help of an electroscope. The activity is compared with the added element. The weight of the element in the unknown sample can then be calculated.
7. Use of the independent method of analysis: Sometimes the complete analysis has to be carried out in an entirely different manner to get accurate results. e.g. strength of HCl may be determined by two methods:
- Titrating it with a standard solution of a strong base.
- Precipitation with AgNO3 and weighing as AgCl.
If the results obtained by the two methods are in good agreement, it may be said that the values are correct within small limits of errors.
8. Internal Standards: It involves the addition of a fixed amount of a reference material, i.e. the internal standard to a series of known concentrations of the sample to be determined. The ratios of the physical value of the internal standard and the series of known concentrations are plotted against the concentration values. It should give a straight line. Any unknown concentration can then be determined by adding the same quantity of internal standard and finding where the ratio obtained falls on the concentration scale. The method is of particular interest in spectroscopic and chromatographic determinations.
Make sure you also check our other amazing Article on : How do you prepare 0.1 M disodium edetate solution?
